Finding Value in Digital Therapeutics During COVID-19
As COVID-19 continues to impact how populations with chronic diseases and mental health conditions across the world are living and receiving healthcare, it is important to consider how the use of a new category of medicine may address some of the emerging challenges – and increasingly growing pressures – introduced by the new realities of life created by COVID-19.
First, what is a digital therapeutic?
Digital therapeutics (DTx) deliver therapeutic interventions directly to patients using evidence-based, clinically evaluated software to treat, manage, and prevent a broad spectrum of behavioral, mental, and physical diseases and disorders. These products are not intended to directly treat COVID-19.
DTx products may be used independently or in tandem with remote or in-person clinician-delivered therapy to optimize patient outcomes. They differ from lifestyle and wellness apps, adherence, diagnostic, and telehealth products.
Digital therapeutics undergo clinical trials, collect real world outcomes, and are based on patient-centered core principles and product development best practices, including product design, usability, data security, and privacy standards.
How could DTx products address prevailing concerns resulting from COVID-19?
Patients with chronic diseases are facing new challenges in the midst of the COVID-19 pandemic. These individuals still need to manage their conditions, but no longer have as much flexibility in seeing their healthcare providers, picking up and managing their medications, exercising, and eating well-balanced meals, among many other things. The current burden of COVID-19 exacerbates how patients are able to self manage their conditions due to physical distancing, isolation, and complications in accessing healthcare services.
What value could DTx products bring to this situation?
- Increase remote access to therapies that are clinically demonstrated as safe and effective.
- Provide care independent of a patient’s schedule and in the safety of their home environment.
- Are easily scalable and often accessible through patient-owned devices (e.g., smartphones, tablets).
- Generate actionable, real world insights that enable intelligent data-driven care management and clinical decision making.
- Have the potential to measure the use of medications and consumables (e.g. glucose test strips), and use this information for automatic home-delivery refills, thus avoiding additional trips to the store.
- DTx product examples include:
- DarioHealth’s product for type 1 and type 2 diabetes has shown that patients experienced reduced hypoglycemic events by 50% and high glycemic events by 40% over a 2-year period.
- BlueStar, Welldoc’s product for type 1 and type 2 diabetes, aids patients in diabetes self-management in the home and clinical settings, in addition to delivering 1.7 – 2.0 A1c reductions in clinical and real-world studies.
- Kaia Health’s DTx product enables chronic pain sufferers to self-manage their condition and associated psychosocial aspects at home using only a smartphone – increasing healthcare access during this transitionary period for many workforces. Users showed a 43% pain level decrease compared to controls in its latest randomized controlled trial (RCT).
Patients with anxiety, depression, cognitive impairments, and other mental health conditions are also facing new challenges in the midst of the COVID-19 pandemic. With an increased focus on social distancing, people are facing long periods of quarantine, social isolation, and uncertainty. These conditions are often significantly impacted by stress, and children and adults will likely not receive the stimulation they normally would for long periods of time. Healthcare systems do not currently have the capacity to provide evidence-based therapies to all of the individuals dealing with mental health conditions and disorders. This represents a high degree of unmet medical need.
What value could DTx products bring to this situation?
- Enable the expansion of care outside of a traditional clinic setting and offer treatments for non-communicable diseases without requiring physical patient contact.
- Provide ubiquitous access to treatment options for conditions that previously have been untreated or undertreated by traditional medications and therapies.
- Provide patients, caregivers, and clinicians with secure progress updates.
- DTx product examples include:
- SilverCloud Health’s studies have shown in university students a 25-45% improvement in depression, anxiety and stress scores at 3 months, and an RCT in a routine care setting showing lasting positive effects on depression, anxiety, and functional impairment up to 12 months after start of treatment.
- Big Health’s product, Sleepio, demonstrated in a placebo-controlled RCT and 11 additional studies that 76% of users achieved healthy sleep levels. Health economic data also suggest that Sleepio is associated with reductions in health care costs.
- Happify’s RCT found a 25% reduction in both the symptoms of anxiety and depression for patients when compared with an active comparison condition, pyschoeducation, and used as directed by this study.
- Palo Alto Health Sciences’ product, Freespira, has demonstrated success with these patient populations, showing that after 4 weeks of at-home treatment, more than 80% of patients were panic attack free. Further, 88% of patients with PTSD had a statistically significant reduction in the CAPS 5 score (validated PTSD assessment) at 2 months post-treatment.
- Akili Interactive’s candidate DTx product is designed to improve attention in children using video game-based experiential medicine, showing a statistically significant improvement in objective measures of attention in a randomized, controlled trial of 348 children with ADHD (Attention-Deficit/Hyperactivity Disorder).
- reSET and reSET-O from Pear Therapeutics increase access for patients to Substance Use Disorder and Opioid Use Disorder treatments. Without therapeutic interventions, these patients risk feeling isolated and may revert to using addictive substances.
Healthcare systems are searching for ways to reduce unnecessary clinic visits and hospitalizations for non-acute conditions. If the environmental conditions necessitated by COVID-19 push patients with mental health and chronic disorders into uncontrolled states, these individuals are more likely to require immediate care, thus delivering an additional strain on the healthcare system. Additionally, for patients over the age of 70, this presents an increased likelihood of them being exposed to the virus, infecting others, or demonstrating worse clinical outcomes.
What value could DTx products bring to this situation?
- Increase therapy access without potentially requiring an equivalent workforce expansion.
- Optimize patient outcomes and prevent exacerbations, thus reducing chronic disease-related hospitalizations.
- Potentially allow patients to communicate directly with clinicians to avoid unnecessary in-person visits and allow for direct responses to patient concerns.
- Decrease the economic burden of medical conditions by reducing overall costs.
- DTx product examples include:
Patient Access
In order to address growing mental health needs, provide remote care for patients with chronic conditions, limit viral exposure for aging populations, and reduce unnecessary burdens on clinicians and health systems, it is crucial for patients to be given access to evidence-based digital therapies from the safety of their home environment.
National coverage frameworks for digital health that include DTx products are currently being implemented in Germany and Belgium, while France and the United Kingdom have been financing DTx products regionally for more than three years.
In the United States, DTx products most frequently receive third-party payment by employers, PBMs, or insurers. Unfortunately, digital therapeutics currently are not reimbursed by Medicare or most state Medicaid programs.
Conclusion
This is a challenging and disruptive time for everyone. As we all take the steps necessary to keep our loved ones and ourselves healthy, safe, and cared for, it is important to consider technologies that are available to provide accessible treatments wherever a patient is located, without creating an additional burden on medical professionals.
Patient access to and utilization of digital therapeutics could improve health outcomes, thereby reducing chronic disease-related hospitalizations, mitigating additional pressures on healthcare providers during the COVID-19 outbreak, and reducing vulnerable populations’ potential coronavirus exposure.
About DTA
Founded in 2017, the Digital Therapeutics Alliance (DTA) is a non-profit trade association of industry leaders and stakeholders engaged in the evidence-driven advancement of digital therapeutics. DTA has over 35 global members, located in 15 countries across four continents.
DTA does not function as a certification, accreditation, or standard setting body. DTA does not endorse specific digital therapeutic (DTx) products, nor does information provided through DTA intend to serve as patient-specific medical advice.
DTA Announces 2020 Board of Directors
The Digital Therapeutics Alliance (DTA) is pleased to welcome the following individuals to the DTA Board of Directors:
- Peter Hames, Co-Founder and CEO, Big Health
- Anand Iyer, Chief Strategy Officer, WellDoc
- Pierre Leurent, Founder and CEO, Voluntis
- Eddie Martucci, CEO, Akili Interactive
- Debra Reisenthel, Founding CEO, Board Member, Palo Alto Health Sciences
- Joris Van Dam, Head of Digital Therapeutics, Novartis
- Chris Wasden, Head of HappifyDTx, Happify Health
- Megan Coder, Executive Director, Digital Therapeutics Alliance
“When DTA launched in 2017, the conversation was focused on how digital therapeutics would be defined and work alongside other evidence-based therapies. Through the work of DTA’s Board and members, the conversation has evolved to: ‘How do these products address underserved populations and conditions? What clinical interventions and outcomes are delivered in real world settings? How is value achieved and quantified for patients, clinicians, and payors?’,” shared Megan Coder, DTA’s Executive Director.
Over the last two years, DTA released a series of foundational documents to differentiate digital therapeutics (DTx) from other products on the market and establish a level of rigor all DTx products should achieve. Publications include:
Individuals who are elected by DTA members to the Board of Directors serve two-year terms. In line with DTA’s 2020 priorities, the Alliance will launch two additional Work Groups and appoint Special Advisors to provide guidance related to strategic initiatives.
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding and adoption of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors in order to improve clinical and health economic outcomes. To learn more please visit: www.dtxalliance.org.
Digital Therapeutic Industry Leaders Promote Ethical Standards and Product Development Best Practices
Trust is crucial as patients increasingly use digital therapeutic products for the delivery of high quality, evidence-based medical treatments
Washington, D.C. — Today, the Digital Therapeutics Alliance (DTA) released an Industry Code of Ethics, a series of Best Practices, and an updated product claims categorization chart for digital therapeutic (DTx) products. DTA is also proud to collaborate this week with the Digital Medicine Society, HealthXL, and NODE.Health to release a broad categorization of the Digital Health, Digital Medicine, and Digital Therapeutic industries.
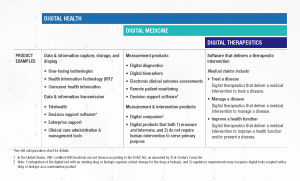
DTx products deliver personal and adaptive medical interventions to patients and are driven by high quality, evidence-based software programs. As such, they make claims to prevent, manage, or treat a medical disease, and therefore carry a higher level of risk than other products in the broader digital health landscape. Digital therapeutics do not include lifestyle and wellness apps, clinical decision support, or products that deliver static, automatic interventions to patients.
“DTx products provide a monumental opportunity to improve how healthcare is delivered by increasing therapeutic options for unmet medical needs and expanding patient access and engagement. Given the importance of these therapies, DTA members appreciate the responsibility of protecting patients and end users every step of the way,” shares Megan Coder, DTA Executive Director. “In many ways, digital therapeutics are an extension of a clinician in a patient’s everyday life as they receive active treatment outside of a clinic. This is why these documents are so important.”
In order to reinforce the foundations of the digital therapeutic industry, DTA is proud to release the following:
- DTx Code of Ethics: As the DTx industry continues to expand and deliver medical interventions directly to patients, it is important for end users to know that the companies working to develop, evaluate, and market digital therapeutics are acting in the best interest of patients. Therefore, DTA member companies collaborated with fellow leaders in the healthcare industry to develop a DTx Industry Code of Ethics. This Code demonstrates DTA Member Companies’ dedication to developing and bringing DTx products to market in a responsible way.
- Industry Best Practices: Last year, DTA published Industry Core Principles to which all DTx products should adhere. These principles address topics such as robust product quality and design, privacy and security, end user engagement, and clinical evaluation. In order to provide better clarity for patients, clinicians, and payors on how to assess product alignment with these principles, DTA compiled a series of Best Practices that products may utilize to demonstrate robust design, evaluation, and delivery of interventions.
- DTx Product Claims: In 2018, DTA published an Industry Report that provided the definition of a DTx product, industry principles, and a chart representing DTx product claim types. In order to provide a more appropriate representation of the industry, DTA recently developed a more streamlined approach to the DTx Product Claims categorization.
- Industry Categorization: Clarity and trust are crucially important in this quickly evolving industry. With this mission, DTA collaborated with other organizations to outline the landscape of digital health products available to end users and clinicians. This Digital Health Industry Categorization demonstrates how products making higher-risk medical claims must undergo greater levels of clinical evidence and regulatory oversight.
“Currently there is tremendous confusion among patients, clinicians, and payors about the types of products that exist across the broader digital health landscape,” said Coder. “It is important for patients and other users to understand what types of digital products they are using, what products’ primary functions are, and the levels of clinical outcomes and regulatory oversight that are required of them.”
These resources demonstrate that not all digital products are the same, and those products that are delivering medical interventions and making medical claims are taking a serious approach to ensuring patient safety, protection, and care.
Access these materials on https://dtxalliance.org/aboutdtx/.
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding and adoption of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payers in order to improve clinical and health economic outcomes. To learn more please visit: www.dtxalliance.org.
Updated: 13 November 2019
Digital Health, Digital Medicine, Digital Therapeutics (DTx): What’s the difference?
Clarity matters. Here’s what you need to know.
Digital health products have become integral to the prevention, diagnosis, treatment, and management of health and disease. Consumers rely on digital health apps to improve their focus, track their fitness, and optimize their wellbeing. Clinicians use digital health products to gain insights on patient outcomes, conduct telehealth visits, and treat aspects of diseases otherwise unaddressed by traditional medications.
In theory, consumers and clinicians know that there is a difference between these products. But in practice, how do they differentiate between products that accurately collect health information versus those that do not, or those that provide clinicians with informational versus actionable insights?
Not all digital health products are the same. But what are their differences? How should each product be used? What should expectations be about product outcomes? How much evidence is needed to go to market? What type of clinical evidence or regulatory oversight is necessary?
NICE’s evidence standards framework for digital health technologies and the eHSG (European Commission eHealth Stakeholder Group) Guidance, both released this year, address varying requirements within the digital health universe. With the industry garnering such support, the need to categorize and differentiate between its various solutions becomes even more important.
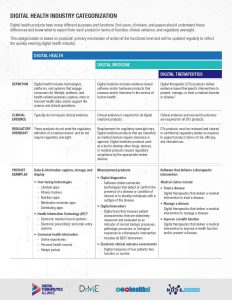
Providing clarity and trust is crucial in this quickly evolving industry. With this as our mission, the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health are collaborating to describe the landscape of digital health products available to end users and clinicians, in addition to the level of clinical evidence and regulatory oversight that correlates to each product category.
As these transformative technologies are laying the groundwork for a new form of healthcare, we must ensure that the digital tools we are placing our trust in are indeed worthy of that trust.
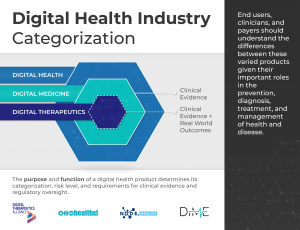
Product risk matters
Digital Health is a broad category that encompasses Digital Medicine, which in turn includes Digital Therapeutics. Products in these categories make different levels of claims and therefore have different levels of risk. As such, they have varying requirements for clinical evidence and regulatory oversight.
In parallel industries such as transportation, higher levels of research and regulation are required for higher risk functions (e.g., operating autonomous vehicles) than lower risk functions (e.g., bluetooth enabled hands-free car kits). Similarly, end users and clinicians should expect that higher levels of evidence and oversight are required for digital health products that make higher risk claims.
Thus, the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health have collaborated to propose this risk-based framework so end users may discern between products of different risk and corresponding levels of necessary evidence and regulatory oversight.

Why categorize the digital health industry?
Rock Health, the first venture fund dedicated to digital health, defines digital health as the space at the intersection of technology and healthcare. HealthXL shares this broad perspective and serves the entire and extensive field of digital health through its market intelligence platform and expert community.
DiMe focuses on digital medicine, the field of evidence-based digital health tools that measure and/or intervene in the service of health to support the practice of medicine broadly, including treatment, recovery, disease prevention, and health promotion for individuals and across populations.
NODE.Health is a membership-based organization with a mission to bring the rigor of evidence-based science to digital medicine. NODE.Health builds on and explores the knowledge base now required to lead global healthcare systems and industry into the value-based era of digital transformation in health.
DTA is the industry association advancing the field of digital therapeutics (DTx). DTx products employ high quality software to deliver evidence-based therapeutic interventions that prevent, manage, or treat a broad spectrum of physical, mental, and behavioral conditions.
These distinctions are intended to clarify, not complicate, the field of digital health. We agree with our FDA and NIH colleagues that:
Effective, unambiguous communication is essential for efficient translation of promising scientific discoveries into approved medical products. Unclear definitions and inconsistent use of key terms can hinder the evaluation and interpretation of scientific evidence and may pose significant obstacles to medical product development programs.
We define these categories to support:
- Consumers of digital health tools — health systems, clinicians, researchers, payers, and patients — seeking to select the most fit-for-purpose digital products for their needs.
- Developers and manufacturers of digital health tools in pursuing the most appropriate go-to-market strategies for their products.
Specifics are invaluable
The digital health field is not yet served by a standard lexicon, and the many disciplines that comprise digital health are often separated by a common language. Anyone who has ever been part of a conversation about ‘validation’, for example, knows that every discipline has its own definition.
This categorization framework goes beyond the existing definitions of digital health, digital medicine, and digital therapeutics to provide clarity with examples of products and product types.

The digital health universe is rapidly expanding — both in size and capabilities. Although this categorization is not intended to be wholly comprehensive, DiMe, DTA, HealthXL, and NODE.Health commit to regular framework updates to ensure it evolves with the industry and remains a valuable tool for all stakeholders.
How to use this framework
This framework is not intended to assign hierarchy to the different digital health product categories. No category is more or less valuable to the advancement of health, healthcare, and/or health research; they are simply governed by different evidence and regulatory requirements.


In addition, users of this framework should not assume that digital health products have no degree of risk compared to digital medicine products or digital therapeutics. While the latter two are subject to higher evidence requirements and regulatory oversight due to greater clinical risk, commercial digital health tools may pose their own unique risks.
For example, many digital health products may boast robust evidence despite its not being required, and the absence of such requirements can make it challenging to identify ‘digital health’ apps that have been proven effective. Similarly, there may be unintended consequences associated with the use of digital products without a robust body of evidence attesting to their safety, efficacy, and equality of performance.
Digital tools falling into the digital health category are currently governed by caveat emptor, or “buyer beware”. This is particularly challenging in an industry plagued by information asymmetry.
Further, privacy and security concerns transcend this framework’s categorical boundaries of digital health, digital medicine, and digital therapeutics. Even when the necessary clinical evidence is available for a digital health product, other considerations such as security features, ethical approaches to data rights and governance, and economic feasibility should be considered.
Why now?
There is a pressing need for further work to better characterize digital health products based on holistic assessments of risk.This is necessary to support trust in digital health, digital medicine, and digital therapeutics, and from there, realize their potential to improve health, healthcare, and health research.
By using this categorization framework, we trust that: patients will better understand what digital products they are using and for what purpose, clinicians will better leverage digital health products in practice and know how they relate to other treatments, payers will better understand the landscape of products available to patients and the type of value and clinical outcomes they should expect, and pharma will see the R&D requirements and commercial opportunities each offers.
Wellness products deliver different outcomes than diagnostic products. Adherence products require different types of evidence and regulation than therapeutic products. Clinical decision support tools support clinicians differently than telehealth products.
Clarity matters. And together our organizations are glad to be part of this conversation.
Readers: We value your feedback, and encourage you to submit your comments on the framework here.
________
Authors: Jennifer Goldsack, Megan Coder, Chandana Fitzgerald, Natalie Navar-Mattingly, Andy Coravos, and Ashish Atreja, MD
________
The Digital Medicine Society (DiMe) is a Massachusetts non-profit and professional society for individuals from all backgrounds working to advance digital medicine to optimize human health.
The Digital Therapeutics Alliance (DTA) is a non-profit trade association with the mission of broadening the understanding and integration of clinically-evaluated digital therapeutics into healthcare to improve clinical and health economic outcomes.
HealthXL is the market intelligence platform and expert community for digital health.
NODE.Health is a 501(c)(3) non-profit organization dedicated to education, validation and dissemination of evidence based digital medicine.
DTA Launches Payer Advisory Group
The Digital Therapeutics Alliance is pleased to launch our Healthcare Payer Advisory Group. We are seeking global engagement from individuals who have experience working within healthcare decision maker and payer settings.
Advisory Group members will be invited to participate in quarterly meetings and engage in relevant Alliance initiatives. Interested individuals are encouraged to apply by September 15.
This follows the establishment of DTA’s Healthcare Clinician Advisory Group earlier this year, which brings together physicians, nurses, and pharmacists from a variety of countries and healthcare settings.
The MM&M Podcast 5.9.2019: Digital Therapeutics Alliance’s Megan Coder
Recorded live from the MM&M Transforming Healthcare conference at the Edison Ballroom in midtown Manhattan, this episode of the podcast features Megan Coder, executive director of the Digital Therapeutics Alliance. She and MM&M’s executive editor Marc Iskowitz talk about the leading firms in digital therapeutics, what the relationship is between DTx firms and pharma/payers and how those in the discipline are using real world evidence studies to prove their value.
Digital Therapeutics Need Quality Standards
Forbes (Greg Licholai) – Quality standards are necessary to help promote adoption of the novel class of interventions called digital therapeutics. This rapidly emerging industry needs to bring together regulators, engineers, practitioners, and companies to agree on definitions that enable innovation, according to product development and regulatory specialists.
As the digital transformation sweeps through healthcare, industry experts say that new classes of medicines and therapeutics present extremely promising opportunities.
2019 DTA Board of Directors
| The DTA Board of Directors has done a fantastic job both founding and leading the organization over this past year.
In December 2018, DTA hosted an election to add two new members to the board. We are therefore pleased to announce our newest Directors:
- Peter Hames, Big Health
- Joris van Dam, Novartis
They will be joining current board members:
- Anand Iyer, WellDoc
- Pierre Leurent, Voluntis
- Eddie Martucci, Akili Interactive
- David Van Sickle, Propeller Health
- Alex Waldron, Pear Therapeutics
|
Digital Therapeutics Alliance Establishes Foundational Definition and Industry Core Principles
ARLINGTON, VA – October 29, 2018 – Digital therapeutics (DTx) – a new category of medical interventions – combine patient-centric technologies with evidence-based medicine to deliver highly personalized care. Digital therapeutics present patients, healthcare providers, and payers with evidence-based technologies that have the ability to elevate medical best practices, address unmet medical needs, expand healthcare access, and improve clinical and health economic outcomes.
Today, the Digital Therapeutics Alliance (DTA) released a comprehensive report that provides an overview of the DTx industry and establishes the definition of a digital therapeutic. Core principles and best practices related to the design, manufacture, clinical validation, and regulatory oversight of these products are also featured. Visit www.dtxalliance.org/dtx-solutions to access the report.
“The integration of digital therapeutics into healthcare is no longer a theoretical conversation. As an Alliance, we are focused on building a strong foundation for this rapidly growing industry,” said Megan Coder, DTA Executive Director. “We are committed to empowering patients, healthcare providers, and payers with engaging and accessible tools that deliver meaningful clinical outcomes across a broad spectrum of physical, mental, and behavioral disorders.”
Digital therapeutics deliver evidence-based therapeutic interventions to patients that are driven by high quality software programs to prevent, manage, or treat a medical disorder or disease. They incorporate advanced technology best practices and are validated by regulatory bodies as required to support product claims regarding risk, efficacy, and intended use.
Over the coming months, DTA will further develop best practices and frameworks that directly support the design, validation, utilization, and regulatory oversight of digital therapeutics across multiple cultures, languages, and national borders.
The Digital Therapeutics Alliance
The Digital Therapeutics Alliance is a global non-profit trade association with the mission of broadening the understanding, adoption, and integration of clinically-validated digital therapeutics into healthcare through education, advocacy, and research. DTA envisions enabling broad access to high quality, evidence-based digital therapeutics for patients, healthcare providers, and payers in order to improve clinical and health economic outcomes. For more information, please visit: www.dtxalliance.org.
Digital Therapeutics Alliance boosts European presence
DUBLIN, IRELAND – 16 October 2018 – S3 Connected Health News –
- Digital Therapeutics Alliance (DTA) boosts Europe presence to advance the adoption of evidence-based digital therapeutics
- S3 Connected Health, a DTA member, is working alongside fellow EU-based members to lead efforts to integrate digital therapeutics into European healthcare
- DTA engages with patients, providers, payers, and regulators to guide and scale the global digital therapeutics (DTx) industry
The Digital Therapeutics Alliance (DTA) has expanded its European membership by announcing that S3 Connected Health is joining the industry body.
DTA is a global non-profit trade association which aims to broaden the understanding, adoption, and integration of clinically-validated digital therapeutics in healthcare through education, advocacy, and research.
Launched in October 2017, DTA is committed to engaging with patients, providers, payers and regulators to define and develop meaningful resources to guide and scale adoption of digital therapeutics (DTx). It works with industry leaders to aid the introduction of tested and trustworthy digital solutions in healthcare. The Alliance has now grown its global membership to 20 companies, bringing together wide-ranging expertise from firms engaged in implementing digital therapeutics in drug discovery and clinical pathways.
In the US, DTA supports the work of the US Food and Drug Administration (FDA) and related efforts to drive regulatory, clinical, organizational and financial integration of digital therapeutics into mainstream healthcare. The expansion of its European presence will enable the Alliance to progress parallel efforts across the diverse and varied European healthcare ecosystem.
S3 Connected Health specializes in the design and development of digital therapeutics and digital health solutions. Focused on addressing the problem of therapy management and medication non-adherence, which is estimated to account for hundreds of thousands of premature deaths globally each year, costing the pharmaceutical industry around $637bn in lost revenues and compromising positive patient health outcomes.
Having pioneered successful, clinically-validated telehealth solutions in the UK and Italy over the past decade, S3 Connected Health has invested heavily in its Affinial platform, a solution-as-a-service that uses behavioral science techniques and user data to deliver individualized support to help patients to manage their therapy and remain adherent to medications across multiple conditions.
S3 Connected Health will bolster DTA’s presence in Europe and provide a platform for increased understanding of the regulatory, legal and reimbursement pathways on behalf of digital therapeutics firms within institutions including the European Medicines Agency.
Megan Coder, DTA Executive Director, said:
“DTA members are dedicated to advancing novel digital therapeutics aimed at transforming patient clinical outcomes and adding value to patients, caregivers, healthcare providers, and payers. Convening thought leaders from our growing membership and industry partners allows DTA to receive critical input from a wide array of stakeholders in order to establish a strong foundation for this robust and rapidly growing industry.”
Jim O’Donoghue, President, S3 Connected Health, said:
“S3 Connected Health’s expertise in developing and delivering Digital Therapeutics and Digital Health Solutions will help the DTA advance key aspects of the DTx industry.
“Digital therapeutics offer huge potential to dramatically improve patient healthcare outcomes, and we must ensure that this opportunity is realized by engaging with industry stakeholders for effective adoption and integration of DTx in mainstream healthcare, clinical and reimbursement pathways.
“This will enable DTx companies to provide solutions that are able to understand the patient context and provide the right treatment for them — in a way that can be personalized, relevant and context-aware.”
###
About S3 Connected Health
S3 Connected Health design and develop digital therapeutics, connected patient care solutions and clinical decision support software. We personalize support and interventions to enable better clinical care, empower patient self-management and improve healthcare outcomes.
For more information on developing patient support programs with S3 Connected Health, visit www.s3connectedhealth.com.
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association with the mission of broadening the understanding, adoption, and integration of clinically-validated digital therapeutics into healthcare through education, advocacy, and research. To learn more please visit: www.dtxalliance.org.