Clarity matters. Here’s what you need to know.
Digital health products have become integral to the prevention, diagnosis, treatment, and management of health and disease. Consumers rely on digital health apps to improve their focus, track their fitness, and optimize their wellbeing. Clinicians use digital health products to gain insights on patient outcomes, conduct telehealth visits, and treat aspects of diseases otherwise unaddressed by traditional medications.
In theory, consumers and clinicians know that there is a difference between these products. But in practice, how do they differentiate between products that accurately collect health information versus those that do not, or those that provide clinicians with informational versus actionable insights?
Not all digital health products are the same. But what are their differences? How should each product be used? What should expectations be about product outcomes? How much evidence is needed to go to market? What type of clinical evidence or regulatory oversight is necessary?
NICE’s evidence standards framework for digital health technologies and the eHSG (European Commission eHealth Stakeholder Group) Guidance, both released this year, address varying requirements within the digital health universe. With the industry garnering such support, the need to categorize and differentiate between its various solutions becomes even more important.
Providing clarity and trust is crucial in this quickly evolving industry. With this as our mission, the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health are collaborating to describe the landscape of digital health products available to end users and clinicians, in addition to the level of clinical evidence and regulatory oversight that correlates to each product category.
As these transformative technologies are laying the groundwork for a new form of healthcare, we must ensure that the digital tools we are placing our trust in are indeed worthy of that trust.
Product risk matters
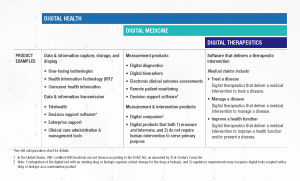
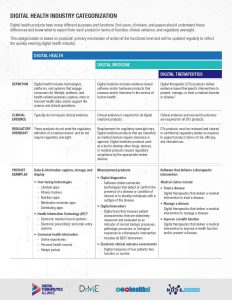
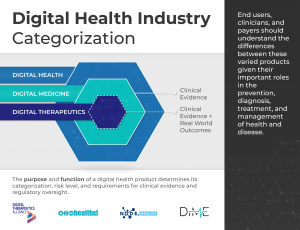
Digital Health is a broad category that encompasses Digital Medicine, which in turn includes Digital Therapeutics. Products in these categories make different levels of claims and therefore have different levels of risk. As such, they have varying requirements for clinical evidence and regulatory oversight.
In parallel industries such as transportation, higher levels of research and regulation are required for higher risk functions (e.g., operating autonomous vehicles) than lower risk functions (e.g., bluetooth enabled hands-free car kits). Similarly, end users and clinicians should expect that higher levels of evidence and oversight are required for digital health products that make higher risk claims.
Thus, the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health have collaborated to propose this risk-based framework so end users may discern between products of different risk and corresponding levels of necessary evidence and regulatory oversight.

Why categorize the digital health industry?
Rock Health, the first venture fund dedicated to digital health, defines digital health as the space at the intersection of technology and healthcare. HealthXL shares this broad perspective and serves the entire and extensive field of digital health through its market intelligence platform and expert community.
DiMe focuses on digital medicine, the field of evidence-based digital health tools that measure and/or intervene in the service of health to support the practice of medicine broadly, including treatment, recovery, disease prevention, and health promotion for individuals and across populations.
NODE.Health is a membership-based organization with a mission to bring the rigor of evidence-based science to digital medicine. NODE.Health builds on and explores the knowledge base now required to lead global healthcare systems and industry into the value-based era of digital transformation in health.
DTA is the industry association advancing the field of digital therapeutics (DTx). DTx products employ high quality software to deliver evidence-based therapeutic interventions that prevent, manage, or treat a broad spectrum of physical, mental, and behavioral conditions.
These distinctions are intended to clarify, not complicate, the field of digital health. We agree with our FDA and NIH colleagues that:
Effective, unambiguous communication is essential for efficient translation of promising scientific discoveries into approved medical products. Unclear definitions and inconsistent use of key terms can hinder the evaluation and interpretation of scientific evidence and may pose significant obstacles to medical product development programs.
We define these categories to support:
- Consumers of digital health tools — health systems, clinicians, researchers, payers, and patients — seeking to select the most fit-for-purpose digital products for their needs.
- Developers and manufacturers of digital health tools in pursuing the most appropriate go-to-market strategies for their products.
Specifics are invaluable
The digital health field is not yet served by a standard lexicon, and the many disciplines that comprise digital health are often separated by a common language. Anyone who has ever been part of a conversation about ‘validation’, for example, knows that every discipline has its own definition.
This categorization framework goes beyond the existing definitions of digital health, digital medicine, and digital therapeutics to provide clarity with examples of products and product types.

The digital health universe is rapidly expanding — both in size and capabilities. Although this categorization is not intended to be wholly comprehensive, DiMe, DTA, HealthXL, and NODE.Health commit to regular framework updates to ensure it evolves with the industry and remains a valuable tool for all stakeholders.
How to use this framework
This framework is not intended to assign hierarchy to the different digital health product categories. No category is more or less valuable to the advancement of health, healthcare, and/or health research; they are simply governed by different evidence and regulatory requirements.


In addition, users of this framework should not assume that digital health products have no degree of risk compared to digital medicine products or digital therapeutics. While the latter two are subject to higher evidence requirements and regulatory oversight due to greater clinical risk, commercial digital health tools may pose their own unique risks.
For example, many digital health products may boast robust evidence despite its not being required, and the absence of such requirements can make it challenging to identify ‘digital health’ apps that have been proven effective. Similarly, there may be unintended consequences associated with the use of digital products without a robust body of evidence attesting to their safety, efficacy, and equality of performance.
Digital tools falling into the digital health category are currently governed by caveat emptor, or “buyer beware”. This is particularly challenging in an industry plagued by information asymmetry.
Further, privacy and security concerns transcend this framework’s categorical boundaries of digital health, digital medicine, and digital therapeutics. Even when the necessary clinical evidence is available for a digital health product, other considerations such as security features, ethical approaches to data rights and governance, and economic feasibility should be considered.
Why now?
There is a pressing need for further work to better characterize digital health products based on holistic assessments of risk.This is necessary to support trust in digital health, digital medicine, and digital therapeutics, and from there, realize their potential to improve health, healthcare, and health research.
By using this categorization framework, we trust that: patients will better understand what digital products they are using and for what purpose, clinicians will better leverage digital health products in practice and know how they relate to other treatments, payers will better understand the landscape of products available to patients and the type of value and clinical outcomes they should expect, and pharma will see the R&D requirements and commercial opportunities each offers.
Wellness products deliver different outcomes than diagnostic products. Adherence products require different types of evidence and regulation than therapeutic products. Clinical decision support tools support clinicians differently than telehealth products.
Clarity matters. And together our organizations are glad to be part of this conversation.
Readers: We value your feedback, and encourage you to submit your comments on the framework here.
________
Authors: Jennifer Goldsack, Megan Coder, Chandana Fitzgerald, Natalie Navar-Mattingly, Andy Coravos, and Ashish Atreja, MD
________
The Digital Medicine Society (DiMe) is a Massachusetts non-profit and professional society for individuals from all backgrounds working to advance digital medicine to optimize human health.
The Digital Therapeutics Alliance (DTA) is a non-profit trade association with the mission of broadening the understanding and integration of clinically-evaluated digital therapeutics into healthcare to improve clinical and health economic outcomes.
HealthXL is the market intelligence platform and expert community for digital health.
NODE.Health is a 501(c)(3) non-profit organization dedicated to education, validation and dissemination of evidence based digital medicine.