DTx Innovation in Europe: 2024 Milestones and a Vision for 2025
Across Europe, advancements in policy, regulation, and reimbursement have set the stage for continued innovation in digital health technologies, despite challenges that demand coordinated action and advocacy.
The events of 2024—marked by regulatory milestones such as the adoption of the AI Act, the European Health Data Space (EHDS), and evolving medical device frameworks—underscore the need for streamlined processes that balance innovation with safety. In this context, the DTA has played a critical role in shaping policies, addressing barriers, and fostering collaboration among stakeholders to support the growth of DTx.
This year, DTA aims to build on these developments by advocating for reforms that enhance regulatory clarity, promote innovation, and expand patient access to life-changing digital therapies.
Read DTA’s Head of European Policy, Emilie Lopes-Fernandes’, thoughts on 2024 and 2025.
The Future of Medicine: Optimizing the Patient Experience with PDURS
The FDA’s recent Prescription Drug Use-Related Software (PDURS) guidance has the potential to transform both the patient and provider experience by integrating digital tools directly with prescription therapies. This advancement allows software to enhance medication use through support for side effect management, adherence, and overall patient engagement, representing a significant step forward in personalized care. For a deeper look into how this guidance could impact the healthcare landscape, read insights from the Digital Therapeutics Alliance’s CEO.
Read Andy Molnar’s PDURS Thought Piece
DTA Releases Final ‘DTx Integration & Workflow Report’
Digital therapeutics (DTx) have the ability to fill gaps in care for people and their families across the world. As a new category of medicine, one of the first barriers is the integration into the traditional healthcare system so that patients can receive access in a way that is convenient and consistent for them. This effort takes significant collaboration amongst diverse parties across the complex healthcare system in the United States.
These parties include policymakers from governing bodies, clinician and provider systems, health plans, pharmacies, DTx product manufacturers, and patients. However, there are many healthcare partners working behind the scenes to make it all work including pricing compendias and EHR vendors. Over the last two years, DTA has worked with these stakeholders in close partnership with NCPDP to address identified pain points in the workflows.
A critical barrier identified is understanding the payment pathways as they impact each stakeholder downstream. Through the generous support of the NCPDP Foundation, DTA was awarded a grant to research how DTx products would be reimbursed. Following the reimbursement workflows, we were able to finish the outline by addressing the distribution of the products into the hands of patients and their families.
We are proud of the efforts of our member task groups and other engaged stakeholders as we present this fourth and final DTx Integration & Workflow Report. While there are recommendations to improve the aspects of the healthcare system, we have been able to achieve our goal to outline how to integrate digital therapeutic products into the standard practice of care.
View final report (September 2024): DTx Integration and Workflow Report
DTA Issues Comment Letter in Response to CMS’ 2025 Proposed Physician Fee Schedule
DTA has officially submitted our comment letter to CMS regarding the 2025 Proposed Physician Fee Schedule. We are excited to support the inclusion of digital mental health treatment (DMHT) codes, a significant step toward increasing the accessibility and adoption of digital therapeutics in healthcare.
This proposal is a major breakthrough in helping Medicare beneficiaries gain access to safe, effective, and scalable digital therapies. As DTx adoption continues to grow, this new policy will pave the way for future innovation and broader reimbursement, helping more people get the care they need.
Read the full comment letter here.
DTA Announces First CME: Understanding the Future of Digital Therapeutics
DTA is proud to present a foundational Continuing Medical Education course designed to educate healthcare professionals about the rapidly evolving field of digital therapeutics. This CME program is designed to provide healthcare professionals with the knowledge and skills necessary to understand, implement, and optimize digital therapeutics in clinical practice.
This course, “Understanding the Future of Digital Therapeutics”, covers a wide range of topics to ensure thorough comprehension of digital therapeutics:
- Describe and define the International Organization for Standardization (ISO) of DTx
- Identify the difference between healthcare professional and patient-facing digital health technologies
- List the criteria for differentiation of categories for digital health therapeutics based on 4 key areas
- Describe which patient populations might be impacted by the ability to access DTx.
Access the CME course for free.
DTA’s 2023 RFI Comment on the Proposed Physician Fee Schedule
September 11, 2023
Via www.regulations.gov
The Honorable Chiquita Brooks-LaSure
Administrator
Centers for Medicare and Medicaid
Department of Health and Human Services
Attention: CMS-1784-P
P.O. Box 8016
Baltimore, MD 21244-8016
RE: Medicare and Medicaid Programs; CY 2024 Payment Policies under the Physician Fee Schedule and Other Changes to Part B Payment and Coverage Policies (CMS-1784-P)
Dear Administrator Brooks-LaSure:
The Digital Therapeutics Alliance (“DTA”) thanks the Centers for Medicare & Medicaid Services (“CMS” or the “Agency”) for the opportunity to provide comments on the Calendar Year (“CY”) 2024 Physician Fee Schedule Proposed Rule (“Proposed Rule”). Our comments focus on CMS’ request for information (“RFI”) to improve the Agency’s understanding of the opportunities and challenges related to the coverage, payment policies, and claims processing of digital therapeutics.
DTA is a 501(c)(6) non-profit trade association of industry leaders and stakeholders dedicated to broadening the understanding and adoption of digital therapeutics into healthcare. DTA is the leading international organization on digital therapeutics thought leadership and education with the mission of enabling expanded access to high-quality, evidence-based digital therapeutics for patients, qualified health care professionals (QHPs), and payors in order to improve clinical and health economic outcomes. With our 100 member companies across 17 countries, DTA champions the advancement of high-quality, evidence-based digital therapeutics. We submit the comments below in support of our members and in furtherance of our mission.
Digital therapeutics, or software-based interventions, have emerged as promising tools in managing various medical conditions, including mental health disorders, substance use disorders, and chronic diseases. The U.S. Food and Drug Administration (“FDA”) regulates digital therapeutics as “Medical Devices”, “Software as a Medical Device” (“SaMD”) or “Software in a Medical Device” (“SiMD”).
Digital therapeutics vary in both their delivery method to patients and distribution channels. Some FDA-approved/cleared digital therapeutics products may be standalone SaMD available through a patient’s existing hardware, such as tablets, watches, and virtual reality goggles, while other digital therapeutics products pair software with dedicated hardware. Distribution channels for digital therapeutics products include directly by QHPs, and by specialty pharmacies and DME suppliers upon prescription. Digital therapeutics utilization resolves many of the access to care barriers for Medicare beneficiaries, including increasing access to care for patient populations where QHPs and specialists are limited, such as rural communities. As CMS acknowledged in the RFI, there is a gap in coverage and reimbursement for digital therapeutics, which has limited beneficiary access to these innovative and convenient treatments. CMS already has authority to cover and reimburse digital therapeutics products under the durable medical equipment (“DME”) and incident-to physician service benefit categories. DTA and its members urge CMS to provide coverage of digital therapeutics products under existing benefit categories immediately and to clarify coverage routes for digital therapeutics products in the CY 2024 Physician Fee Schedule Final Rule.
DTA and its members strongly encourage CMS to take the following steps:
1. Clarify coverage pathways for digital therapeutics products under existing benefit categories. DTA strongly encourages CMS to clarify in the final rule that digital therapeutics products can be reimbursable under the (1) DME and (2) incident-to physician services benefit categories when certain coverage criteria are met.
2. Make technical modifications to the DME and incident-to reimbursement frameworks. DTA strongly encourages CMS to modify the coverage criteria and current reimbursement framework for DME and incident-to benefit categories to accommodate the innovative and diverse characteristics of digital therapeutics products. The revised frameworks for these benefit categories must include the reimbursement structure for administering digital therapeutics products and for the furnishing of digital therapeutics by QHPs in patients’ treatment plans. This will involve setting reimbursement rates based on the type of therapy, its effectiveness, and its impact on patient outcomes.
Reimbursement for Digital Therapeutics within the Durable Medical Equipment (DME) Benefit Category
DTA applauds CMS’ recent groundbreaking decision in April 2023 related to coding and benefit classification, where CMS assigned a distinctive Healthcare Common Procedure Coding System (HCPCS) Level II code under the DME benefit category for AppliedVR’s RelieVRx, a virtual reality apparatus and its linked software. This decision demonstrates CMS’ authority to cover digital therapeutics products under the existing DME benefit category. Nevertheless, the pathway to digital therapeutics coverage under the DME benefit category requires further clarification from CMS. DTA strongly encourages CMS to revise its DME regulations to clarify how Digital therapeutics products can be covered under the DME benefit category. In particular, CMS should:
1. Revise the accreditation standards to account for the unique qualities of digital therapeutics products;
2. Address how digital therapeutics products can be covered under DME coverage policies;
3. Align the DME payment policy to account for the duration of Digital therapeutics products in treatment plans with the understanding that transfer of title is not always warranted for Digital therapeutics products; and
4. Establish criteria within the statutory requirements for DME that would enable appropriate digital therapeutic products to qualify (e.g., eliminating non-statutory limitations such as the ability to be rented or used by multiple patients).
These modifications and clarifications to the DME regulations would encourage the development and facilitation of beneficiary access to many innovative digital therapeutics products like AppliedVR. We strongly encourage CMS to collaborate directly with DTA in amending the DME regulations to provide coverage of digital therapeutics products under existing benefit categories immediately and to clarify coverage routes for digital therapeutics products in the CY 2024 Physician Fee Schedule Final Rule.
Reimbursement for Digital Therapeutics within the Physician Services Benefit Category
For several years, CMS has covered FDA regulated medical devices (e.g., SaMD) that are used as part of diagnostic (e.g. IDXDR) and monitoring (e.g., remote therapeutic monitoring) services under the incident-to physician services benefit category. DTA strongly encourages CMS to use its existing authority to rapidly cover and pay for digital therapeutics, which are SaMD that are used for therapeutic purposes, under the incident-to physician services benefit category, starting with the CY 2024 Physician Fee Schedule Final Rule.
DTA understands that there have been and continue to be coding proposals (e.g., shown in the Public Agenda for past and upcoming CPT Editorial Panel meetings) to consider digital cognitive behavioral therapy and remote therapeutic treatment and other digital therapeutics as incident-to services.6 Assuming these code proposals are adopted, this would provide an appropriate mechanism to facilitate coverage for these devices when furnished incident-to a healthcare practitioner’s service. Likewise, CMS may also use its authority to adopt such coding under the HCPCS system where CMS would establish a separate set of dedicated G-codes to account for when digital therapeutic devices are acquired by a Medicare-enrolled practitioner, and that practitioner then furnishes that device to a patient and manages their treatment.
With any of the coding proposals that CMS implements, DTA strongly recommends a coding solution under the incident-to service benefit category that accounts for:
- Professional services, such as initial patient education, the setup of the device, and ongoing treatment management services;
- The furnishing of devices intended to have a therapeutic effect, rather than limiting the application to devices that have a diagnostic or monitoring role as described in existing diabetic retinopathy or remote physiologic (RPM) and remote therapeutic monitoring (RTM) codes; and
- Costs that QHPs face to furnish digital therapeutics, including the cost of acquiring digital therapeutic products, as direct expenses within CMS’ practice expense methodology.
In many aspects, QHPs utilize digital therapeutics furnished incident-to in a manner consistent with CMS’ treatment of supplies under the direct practice expense methodology. For example, QHPs acquire a digital therapeutics license, which amounts to a practice expense of the QHP in furnishing the digital therapeutics to the patient. Furthermore, these expenses are incurred uniquely for a specific patient’s treatment (rather than as an underlying expense for running the practice), and the license to the digital therapeutics is exclusive to that particular patient and as such, cannot be used by another patient.
CMS may need to adapt the practice expense methodology to account for the various financial structures of digital therapeutics furnished incident-to, such as digital therapeutics that are based on a subscription model. DTA encourages CMS to be flexible in determining the practice expense methodology for the various financial models of digital therapeutics furnished incident-to as the Agency has demonstrated its flexibility with its approach to software-based services in both the office setting and hospital outpatient setting.
Alternatively, if CMS determines that digital therapeutics furnished incident-to should be considered as items of equipment, DTA emphasizes the importance of recognizing that the purchase price represents the cost to the practice for the duration of the digital therapeutics treatment for a particular patient. The treatment duration could span over weeks or months, and as such, the costs should be amortized or apportioned over that time period (as opposed to years which is common for other equipment frequently priced by CMS).
Regardless of whether the digital therapeutics product is used as part of or independently of a clinic visit, it is always furnished by the treating QHP or obtained upon order from the treating QHP. Therefore, DTA urges CMS to cover digital therapeutics under the incident-to physician services benefit category and to adjust the practice expense methodology to account for the financial models of digital therapeutics.
By taking these steps, CMS can establish a clear and comprehensive coverage framework for digital therapeutics, ensuring that Medicare beneficiaries have access to these innovative treatments while maintaining appropriate quality and cost standards.
DTA responses to CMS RFI questions
The rest of our letter contains answers to the inquiries stated in CMS’s request for information. We have organized our responses in a way that ensures coherent and distinct answers to similar queries.
Questions:
The function of clinical practice and the process of making decisions in a clinical context
1. How do QHPs determine which patients might be best served by digital therapeutics? How do QHPs monitor the effectiveness of prescribed interventions, such as, but not limited to, for their patients on an ongoing basis once the intervention has begun?
QHPs employ a systematic and patient-centric approach to identify suitable candidates for digital therapeutics and oversee the effectiveness of the treatment. This approach encompasses several key stages:
- The process begins with QHPs conducting a comprehensive assessment, which includes reviewing the patient’s medical history, current health status, and specific healthcare and mental health needs through physical examinations, standard assessments, interviews, and medical tests. The goal of this evaluation is to identify individuals who may benefit from digital therapeutics.
- Informed consent and patient choice are pivotal aspects of this process. QHPs engage in discussions with patients, explaining the nature, objectives, benefits, and potential risks associated with digital therapeutics. Patients and QHPs collaborate to make informed decisions regarding the integration of digital therapeutics into the treatment plan, taking into account patient preferences.
- Once an agreement is reached, QHPs prescribe a specific digital therapeutic, which could be in the form of an application, a software program, a paired hardware and software digital therapeutics, or a wearable device.
- Patients receive training from the provider or directly from the digital therapeutics manufacturer on how to set up and effectively use the digital intervention, as well as expectations for ongoing appointments for coaching, responding to any questions and monitoring the patient’s progress. Patients are encouraged to provide feedback about their experiences with digital therapy.
- Data generated by digital therapeutics, such as usage patterns, adherence to the treatment, and treatment outcomes, are collected and analyzed to assess its effectiveness, thus allowing QHPs and coaches to assess the patient’s progress toward meeting treatment goals. Data is collected and analyzed under strict patient privacy and HIPAA guidelines.
- A final evaluation takes place when the treatment concludes or specific goals are achieved, aiming to assess the overall effectiveness of the digital therapeutic intervention. For patients with chronic conditions or ongoing healthcare needs, continuous monitoring remains crucial, even after the initial treatment phase concludes. Digital therapeutics can be integrated into long-term maintenance strategies.
It’s important to recognize that the specific approach may vary depending on factors such as the healthcare setting, the type of digital therapeutic, and individual patient needs. However, across all situations, patient involvement, education, and sustained communication are fundamental elements in the successful implementation and monitoring of digital therapeutics.
2. What QHPs and auxiliary staff are involved in furnishing RPM and RTM services, including training patients on its use, and to what extent is additional training or
supervision of auxiliary staff necessary to provide an appropriate for and/or recommended standard of care in the delivery of these services?
We would like to express our gratitude for the opportunity to provide input on the technical aspects concerning RTM and RPM. This includes a critical consideration for the future when there may be HCPCS or CPT codes to encompass monitoring and therapeutic devices, such as digital therapeutic devices. In this future scenario, these devices could be concurrently used by QHPs for services that are deemed reasonable and necessary.
Nevertheless, it’s important to note that the current RTM codes do not encompass the therapeutic aspect or the therapeutic devices described in this communication. This limitation means that the existing codes, as they are currently structured, cannot be applied to digital therapeutics. While we do address some of the technical questions regarding RTM and RPM, we firmly believe that it is of utmost importance for CMS to rectify this coding gap for digital therapeutics devices, especially those that provide therapy alongside monitoring, rather than just focusing on monitoring alone.
RPM and RTM services involve a diverse group of healthcare QHPs and auxiliary staff to ensure their effective deployment and patient training. The extent of additional training or supervision required for auxiliary staff varies based on their roles, patient population needs, and the product itself. Key QHPs include physicians or nurse QHPs responsible for diagnosis and care planning, specialist physicians for condition-specific oversight, and registered nurses involved in patient education and monitoring. Auxiliary staff such as Certified Medical Assistants, healthcare technicians, and medical administrative assistants may assist with device setup, data collection, and administrative tasks.
The training requirements for these roles encompass device familiarity, patient education, and adherence to healthcare regulations. Supervision, periodic retraining, and ongoing education are often essential to maintain a high standard of care. The extent of additional training or supervision needed depends on factors like device complexity and organizational policies. Ensuring clear protocols and adequate training is vital for healthcare facilities to effectively deliver RPM and RTM services, and keeping staff up-to-date with evolving technology and best practices in remote monitoring.
3. How are data that are collected by the technology maintained for recordkeeping and care coordination?
Data collected by digital therapeutics are crucial for recordkeeping and care coordination. Proper management and storage of this data are essential to ensure patient care quality, regulatory compliance, and effective communication among QHPs.
Data that is collected by the digital therapeutics technology is shared with the referring HCP and can be stored in the HCP’s Electronic Health Records (“EHRs”) and made available to the patient.
Here is how these data are typically maintained for recordkeeping and care coordination:
- Many healthcare organizations integrate digital therapeutics data directly into patients’ EHRs. This provides a comprehensive and centralized view of a patient’s health information, including physiologic data from monitoring devices, test results, treatment plans, clinical results & notes. EHRs make it easier for QHPs to access and update patient data, promoting seamless care coordination.
- Data from digital therapeutics often flow into secure, cloud-based platforms specifically designed for digital therapeutics. These platforms offer real-time access to patient data, allowing QHPs to monitor patients remotely and collaborate with other team members. Data security and compliance with health privacy regulations (e.g., HIPAA in the United States) are top priorities in these platforms. Additionally, Business Associate Agreements are in place with vendors who have access to this data.
- Ensuring that digital therapeutics technologies are interoperable with existing healthcare systems is essential for effective care coordination. Data collected should be easily shareable with other QHPs , laboratories, and specialists involved in the patient’s care. Standardized data formats and protocols facilitate this interoperability.
- Digital therapeutics often incorporates alert mechanisms. When certain patient metrics or vital signs fall outside predefined thresholds, the system can send alerts to QHPs. These notifications help in timely intervention and care adjustments.
- Within healthcare organizations, dedicated QHPs may be responsible for managing and communicating patient data collected through digital therapeutics. These teams ensure that the right information is shared with the right QHPs at the right time.
- QHPs often have policies and procedures for data retention and archiving. Patient data collected from digital therapeutics may be stored for a defined period, in compliance with regulatory requirements.
- Digital therapeutics data is not only used for immediate care coordination but also for long-term analysis and reporting. Trends and insights gleaned from this data can inform treatment strategies and healthcare policies.
In summary, digital therapeutics data is stored in electronic health records, secure cloud-based platforms, and interoperable systems. QHPs, patients and digital therapeutics manufacturers play essential roles in ensuring data accuracy, privacy, and timely sharing to support effective care coordination and recordkeeping. Compliance with data security regulations and best practices is paramount in maintaining the integrity and confidentiality of patient data.
RPM & RTM Codes
4. What information exists about how an episode of care should be defined, particularly in circumstances when a patient may receive concurrent RTM or digital CBT services from two different clinicians engaged in separate episodes of care?
Defining an episode of care, especially when a patient concurrently receives RTM or Digital Cognitive Behavioral Therapy (CBT) services from separate clinicians, involves several important considerations. It is crucial to prioritize clear care coordination and effective communication among clinicians to ensure a comprehensive approach to the patient’s well-being. Each episode of care should have well-defined goals, objectives, and care plans, especially when clinicians are providing distinct services or managing various aspects of the patient’s health. A patient-centered approach is essential, emphasizing the importance of considering the patient’s preferences and input when crafting care plans and determining how concurrent services are delivered. For instance, in the case of a patient with PTSD, who may be
receiving multiple treatments including a digital therapeutics product as part of the plan, it is vital to adhere to defined treatment durations, often specified by clinical trials and regulatory clearance, such as the FDA’s 510k clearance, which may, in this case, be a duration of 4 weeks.
Accurate documentation within EHRs or similar systems is of utmost importance. These systems should be designed to support data separation and provide accessibility for the clinicians involved. Establishing mechanisms for data sharing and integration, while respecting patient privacy and obtaining consent, is essential to facilitate informed decision-making and prevent duplication of efforts. Adherence to regional or healthcare system-specific regulatory and legal requirements regarding episode definition and patient data sharing, particularly concerning privacy and data security, is crucial. Billing and reimbursement practices should align with the defined episodes and the services provided, while periodic reviews should assess progress and allow for necessary adjustments. Patient education on the different episodes of care, clinician roles, and care coordination is essential to promote understanding and encourage active patient engagement. Guided by ethical principles such as patient autonomy and beneficence, episode definition should prioritize the patient’s best interests in all decisions. Flexibility and adaptability are key when defining episodes of care, tailoring them to meet each patient’s unique needs while ensuring effective communication, clinician collaboration, and regulatory compliance.
5. What are the advantages and disadvantages of a generic RTM device code, versus specific RTM codes?
6. Would generic device codes undermine or stall progress toward a wider set of specific codes that would provide less ambiguity on reimbursement?
The utilization of generic RTM device codes and specific RTM codes in healthcare billing and coding comes with its own set of advantages and disadvantages. Generic codes offer simplicity, flexibility, and cost-effectiveness by streamlining administrative tasks and adapting to various RTM devices. However, determining reimbursement for generic RTM codes presents challenges when payers are tasked with assessing the intricacy and clinical necessity of particular RTM services. This challenge may lead to claim rejections and appeals and/or reduced and disparate reimbursement rates among payers and QHPs. Moreover, the utilization of generic codes may restrict the availability of detailed data required for healthcare decision-making, resource allocation, and research and may further discourage digital
therapeutics innovation due to their ambiguous reimbursement.
Conversely, specific RTM codes provide comprehensive billing information, improved reimbursement rates, and enhanced capabilities for data analysis. These codes enable more accurate tracking of the utilization and effectiveness of different RTM services. Nonetheless, managing a multitude of specific codes can be administratively intricate and may lead to inefficiencies in the coding and documentation processes.
Whether to employ generic or specific RTM codes largely depends on the unique needs of healthcare organizations, the complexity of their RTM services, and the requirements of payers. The presence of generic codes might discourage the creation of specific codes because of their initial administrative ease. Nevertheless, such administrative ease is likely temporary due to the difficulties of determining reimbursement rates for various digital therapeutics products under the generic codes.
To address these complexities, healthcare organizations, payers, and stakeholders should thoroughly evaluate the ramifications of employing generic codes and strive for a balanced approach that fosters the development of specific codes when deemed necessary. This approach can ensure precise billing and facilitate comprehensive data collection to support well-informed healthcare decision-making.
7. We noted in previous rulemaking that even when multiple medical devices are provided to a patient, the services associated with all the medical devices can be billed by only one practitioner, only once per patient, per 30-day period, and only when at least 16 days of data have been collected. We seek information on the type and frequency of circumstances that involve multiple medical devices and multiple clinicians. How might allowing multiple, concurrent RTM services for an individual beneficiary affect access to health care, patient out-of-pocket costs, the quality of care, health equity, and program integrity?
The RPM and RTM professional work codes (99457, 99458, 98980, and 98981) are not – and should not – be subject to any single provider limitations. To do so, would be akin to limiting Medicare beneficiaries to a single physician for all medical necessities. RPM and RTM professional work via treatment management services should be allowed to be performed and billed/reported by any practitioner or specialist who is working with a single patient, per 30-day period, and not contingent by the 16 days of data collection requirement since that policy was not defined by CMS on codes 99457, 99458, 98980, and 98981 (as described in the
preceding section supra).
It should also be noted that CPT has not set stipulations on the number of physician QHPs and nonphysician QHPs that may concurrently bill 99453 and 99454. It is very possible that more than one provider may be involved in RPM of the same patient, under separate treatment plans, for separate conditions (consider the following):
– Comorbid patient is under the simultaneous care and supervision of the following:
-
- Specialist 1 (Cardiologist) who is remotely monitoring heart failure via various digital medical devices as defined by FDA including a weight scale, a blood pressure cuff monitor, and/or a single lead personal ECG monitor
- Specialist 2 (Endocrinologist) who is remotely monitoring diabetes mellitus via various digital medical devices as defined by FDA including a glucose monitor, and a stand-on temperature monitoring system to assess inflammatory changes associated to foot ulcers
CMS should not suggest that only one provider may bill remote monitoring codes, per patient, per 30-day period. Additionally, there should be no implicit priority between medical specialties. Doing so creates provider confusion, and additional burdens to QHPs who may become reluctant to conduct remote monitoring services.
Data Privacy and Patient Safety
In response to:
8. What standards have interested parties developed or consulted to ensure the physical safety and privacy of beneficiaries utilizing digital cognitive behavioral therapy (CBT) and/or other digital therapeutics for behavioral health?
9. What are some potential considerations for protecting the privacy and confidentiality of the patient population in digital therapeutics, including compliance with State behavioral health privacy requirements?
FDA regulates medical devices using a risk-based framework involving a classification of each device into one of three classes: Class I, II, or III. Regulatory control and requirements increase from Class I (lowest risk) to Class III (highest risk). FDA generally must review marketing applications describing the safety and performance (including relevant testing) for each Class II or III device and provide specific authorization before such devices may be commercialized; however, Class I (and some Class II) devices are exempt from this pre-market review and authorization process. Such pre-market review and authorization process allows FDA to
confirm that the risks associated with each Class II or III device are acceptable in light of the potential medical benefits provided by the device. FDA has classified CBT devices currently on the market at 21 CFR 882.5801 (including CBT for gastrointestinal conditions, substance use disorders, sleep disorders and pain relief) into Class II, and therefore, the agency must review the safety and performance of such devices and ensure that each has an acceptable risk profile and that each manufacturer has implemented appropriate controls to mitigate such risks. Other CBT devices intended to treat or mitigate the symptoms or effects of other specific
diseases or conditions are regulated by FDA as Class II or III medical devices (depending on the associated risks to patients) and require pre-market review and authorization to ensure that any potential risk to a patient’s physical safety is appropriately minimized or mitigated.
QHPs assess patients prior to prescribing a digital therapeutic and are required to treat patients within the standard of care. As with all medical devices and therapeutic interventions, the level of involvement by QHPs to meet the standard of care would depend on the patient and the risks of the specific therapeutic. Notably, digital therapeutics increase a QHP’s capability to monitor, manage and treat patients by:
- Adding a new therapeutic modality – in addition to pharmaceuticals and clinician-delivered therapies – to clinicians’ arsenal of available patient care options;
- Enabling clinicians to ensure the delivery of active clinical care to patients in their home environments between in-person or virtual visits;
- Providing access to actionable data generated during the patient’s use of the digital therapeutics therapy (i.e., frequency, duration, impact of therapy use); and
- Enabling utilization of health coaches and mid-level healthcare workers to support digital therapeutics use, thus freeing up time for other clinicians.
There are multiple factors that drive information privacy and security considerations, ranging from ethical concerns and consumer/customer expectations to regulatory compliance and breach costs. Due to increasing cybersecurity risks and the complexity of the regulatory regime, manufacturers, organizations and other stakeholders often consult with experts in healthcare cybersecurity, data privacy, and ethics to develop and implement effective safety and privacy measures for digital therapeutics. Individually identifiable health information (IHI) is subject to a complex regulatory regime in the United States. At a minimum, consumer IHI is
generally subject to compliance with the privacy and security expectations of the Federal Trade Commission in connection with Section 5 of the Federal Trade Commission Act in some cases multiple state laws that apply to the privacy and security of consumer information (including a breach of security). HIPAA requirements also apply, as well as state laws specifically protecting IHI. For CBT, state laws protecting mental health information can also apply, in addition 42 C.F.R. Part 2 and similar state laws if substance use disorder treatment records are involved (either directly or by contract).
Manufacturers of digital therapeutics publish privacy policies and operate in strict compliance with pertinent privacy laws. This commitment ensures the preservation of beneficiaries’ privacy and the safeguarding of personal data through the implementation of robust cybersecurity safeguards and protocols. Digital therapeutics manufacturers often are under contractual terms with QHPs and healthcare organizations relating to privacy and security of information that are more stringent than legal requirements, in addition to undergoing information security due diligence and audits. As further discussed below, there are multiple standards and frameworks available to address these considerations.
Digital therapeutics meet the definition of a medical device and thus are subject to applicable FDA cybersecurity requirements. HIPAA privacy and security requirements also apply. In addition to FDA and HIPAA regulatory frameworks and guidance, numerous other privacy and security frameworks, standards, and certifications exist, for example:
- National Institute of Standards and Technology
- International Organization for Standardization
- Center for Internet Security Controls
- American Telemedicine Association Health Data Privacy Principles
- HITRUST Alliance Certification
- Better Business Bureau Vendor Privacy Certification
Organizations and stakeholders also frequently consult guidelines relevant to privacy and security considerations, for example various healthcare organizations, such as the American Psychological Association and the World Health Organization have developed guidelines specifically for telemedicine and digital health.
Conclusion
In conclusion, we reiterate our request, stated at the beginning of our RFI responses, that CMS move forward in the CY 2024 PFS Final Rule with defining digital therapeutics coverage and payment pathways within Medicare’s existing DME and incident-to benefit categories.
The Digital Therapeutics Alliance thanks CMS again for the opportunity to comment on the RFI regarding the benefits of digital therapeutics and the current challenges to beneficiary access to digital therapeutics. If you have any questions about these comments, please do not hesitate to contact DTA Director of US Government Affairs Sara Elalamy at sara@dtxalliance.org.
Sincerely,
Andy Molnar
Chief Executive Officer
Digital Therapeutics Alliance
CC: Meena Datta, Partner, Sidley Austin
Elizabeth Hardcastle, Partner, Sidley Austin
Sama Kahook, Associate, Sidley Austin
Legislative Update: New Bill H.R. 8816 Explores Options for Medicare Coverage of Digital Therapies and AI-powered Healthcare
Just three weeks ago during DTA’s Advocacy Day on Capitol Hill, DTA and its Members asked legislators for two things: 1) Co-sponsor the Access to Prescription Digital Therapeutics Act, and 2) Urge CMS to use their authority to expand product supply coding and physician services. DTA is now very excited to see the introduction and immediate mark-up through Ways and Means of H.R. 8816, the American Medical Innovation and Investment Act of 2024. This bill is part of a package intended to increase access for seniors to advanced, cutting-edge medicine and the timing is significant given that CMS is preparing to release the proposed Physician Fee Schedule for 2025.
As part of the Digital Therapeutics Alliance’s policy and legislative strategy to continue to move the industry forward while working on the Access to Prescription Digital Therapeutics Act, H.R. 8816 represents a substantial step forward. Among other things, the bill requires exploration of the options that exist for Medicare coverage of AI-powered health care and digital therapies. Specifically, Section 6 would require CMS by no later than January 1, 2026 to:
- Issue guidance on requirements for coverage of prescription digital therapeutics furnished by a physician or incident to a physician’s service and clarifying when Medicare Advantage plans may cover prescription digital therapeutics as a supplemental benefit; and
- Submit a report analyzing the existing authority of CMS to provide payment for prescription digital therapeutics and describing any additional statutory authority needed to expand such coverage to the Ways and Means Committee, Energy and Commerce Committee, and the Senate on Finance Committee.
The bill emphasizes the importance of modernizing healthcare delivery through innovative digital tools, which could significantly improve patient outcomes and healthcare efficiency.It not only legitimizes digital therapeutics within the broader healthcare framework but also is a critical step towards Medicare coverage for prescription digital therapeutics. This can drive broader adoption of digital health technologies, encourage further innovation, and ensure that patients have access to cutting-edge treatments.
Thank you to Representatives Buchanan, Hern, and Thompson for continuing to support this industry and drive forward access to digital therapeutics.
Read the Bill here: https://www.congress.gov/bill/118th-congress/house-bill/8816/text
DTA Announces DTx Accreditation Program
Collaboration to expand existing DirectTrust programs by establishing a new accreditation program for efficacy and data privacy and security for digital therapeutics applications and platforms
WASHINGTON, DC – June 6, 2024 – DirectTrust®, a non-profit healthcare industry alliance focused on furthering trust in healthcare data exchange through standards, accreditation, and other services, and Digital Therapeutics Alliance (DTA) today announced a collaborative partnership that will provide accreditation for efficacy, data privacy and security for digital therapeutics applications and platforms (DTx).
Through this strategic partnership, DTA and DirectTrust will develop a program set to expand existing DirectTrust programs that provide independent assessment of health apps for privacy, security, and transparency, as well as interoperability compliance with regulations and best practices. DirectTrust will administer the program, while DTA will lead the criteria development.
“This collaboration will add to DirectTrust’s growing suite of programs designed to assess the diverse digital health app and platform market,” said Scott Stuewe, DirectTrust President and CEO. “Our Health App program evaluates privacy and security, while the CARIN Code of Conduct for Consumer-Facing Applications assesses transparency and data use outside of HIPAA. Additionally, our UDAP programs validate effective, scalable connections to national health networks using the FHIR® standard. With the creation of this new accreditation to assess clinical efficacy for Digital Therapeutics, organizations will be able to select the program(s) that are appropriate for their business models.”
Over the past six years, DTA has produced collaborative and validated assets to define and assess DTx products, which will serve as the foundation for program assessment criteria. These assets include a Product Library that incorporates the industry core principles; DTx Value Assessment and Integration Guide; along with the clinical evidence paper “Setting the Stage for a Fit-for-Purpose DTx Evidentiary Standard.”
“As the digital therapeutics industry grows, an increasing number of U.S. clinicians, provider systems, health plans, employers, and patients are evaluating how to best incorporate DTx products into their care plans to provide the highest quality of care,” said Andy Molnar, CEO, DTA. “With an overwhelming number of products touting a wide range of clinical rigor, it is critical that we set a high bar to build trust. In order to achieve this, DTA and DirectTrust are partnering to establish a rigorous accreditation program specific to DTx products that will build upon DirectTrust’s established, and widely adopted, Privacy and Security accreditation program.”
The new program is slated to launch later this year following DirectTrust’s standard procedures of developing proposed criteria and publishing those criteria for a 60-day public comment period.
Call for DirectTrust Criteria Council Sub-Group Members
To establish and codify the criteria to be used for this new program, a focus group comprised of key stakeholders with expertise and interest in clinical efficacy will be established as a subgroup of the DirectTrust Criteria Council. Any interested constituent is welcome to apply to the Criteria Council. Because this group will convene to collaborate on criteria related to Digital Therapeutics accreditation, representation from health care organizations, condition-specific advocacy groups, and digital therapeutics companies is highly encouraged. Already committed to participate in the effort are active participants in the Digital Therapeutics Alliance community including payer representatives Mark Rakowski of Children’s Community Health Plan, Nick Dougerty from Mass General Brigham Health Plan, Jon Vecchiet from OscarHealth, and Jeff Dunn representing Cooperative Benefits Group.
Stakeholders with an interest in participating as a beta organization for accreditation, or in joining the Criteria Council subgroup can complete the form on the DirectTrust website at bit.ly/DTDTx, or email Admin@DirectTrust.org.
DirectTrust’s accreditation and certification programs are governed by the organization’s Electronic Healthcare Network Accreditation Commission (EHNAC). DirectTrust criteria for each of its accreditation programs sets the stakeholder and program specific foundational requirements for assessing an organization’s ability to meet/align with federal and state healthcare reform mandates such as HIPAA/HITECH, 21st Century Cures Act, TEFCA and other mandates and best practices like NIST SP 800-53 800-171, and 800-63, for healthcare organizations focusing on the areas of trust, privacy, security, cybersecurity, breach handling, confidentiality, best practices, procedures, and assets.
About DirectTrust®
DirectTrust® is a non-profit, vendor-neutral alliance dedicated to instilling trust in the exchange of health data. The organization serves as a forum for a consensus-driven community focused on health communication, an American National Standards Institute (ANSI) standards development organization, an accreditation and certification body through EHNAC (the Electronic Healthcare Network Accreditation Commission), and a developer of trust frameworks and supportive services for secure information exchange like Direct Secure Messaging and trusted, compliant document submission.
The goal of DirectTrust is to develop, promote, and, as necessary, help enforce the rules and best practices necessary to maintain privacy, security, and trust for stakeholders across and beyond healthcare. In addition, DirectTrust is committed to fostering widespread public confidence in the interoperable exchange of health information while promoting quality service, innovation, cooperation, and open competition in healthcare. To learn more, visit: DirectTrust.org.
About Digital Therapeutics Alliance (DTA)
As the leading global authority on the evolution of digital health technology, Digital Therapeutics Alliance (DTA) is a 501(c)(6) non-profit trade association of industry leaders and stakeholders dedicated to the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. As the leading international organization on digital therapeutic thought leadership and education, DTA provides the digital health ecosystem with the necessary tools to define, evaluate, and utilize DTx products.
Letter from the CEO: Breaking Bureaucratic Barriers and Navigating the Next Phase of DTx
DTA Members and DTx Stakeholders,
Despite the efforts of entrenched healthcare executives to stifle innovation and prioritize revenue over patient care, we continue to make advancements in providing clinically validated digital healthcare.
I’ve had a unique opportunity to sit down with global organizations, policymakers, investors, and thought leaders over the past three years. As we come out of an incredibly difficult period, I want to share what I have learned as we are clearly moving into the next phase of Digital Therapeutics.
- Why I’m Writing This
I have been swimming through a sea of people saying what is and isn’t possible with DTx, and I have watched the impossible happen and the obvious get shut down. In the U.S., seasoned policy experts with extensive experience on Capitol Hill have said that certain DTx endeavors were unattainable or futile. Initially, I took that as fact, yet, a few months later, they were proven wrong.
I have heard some of our seasoned DTx partners on stage at conferences say “the prescription model will never work”only to be contradicted offstage by a top-ten health system, which exclusively engages with prescription products. (this was 6 months ago). For years now, many have been trying to choose the right business model for the industry, but in digital it is about choosing the right business model for your product. It’s not about jumping on the next buzz word, it’s about being realistic in your approach to the market. For example, Venture capitalists are only hesitant about the prescription DTx model because of inflated projections (seen particularly from Pear Therapeutics) rather than an inherent flaw in the model itself.
- International DTx Landscape
While we have hit speed bumps and roadblocks in the U.S., the global perspective on Digital Therapeutics is strong. In December I sat down with the Korean FDA, and when I asked how I could help, they said the U.S. FDA is visiting to learn from the progress that has been made in South Korea. . Since then, we have seen AIMMED launch Somzz in South Korea, and the Korean FDA said they want to see more DTx come to South Korea as quickly as possible. As U.S. Pharma companies start to embrace PDURS (Prescription Drug Use-Related Software), South Korea is moving quickly with their version, and without the same reimbursement issues faced in the U.S. (South Korea: Digital Medical Products Act Enacted | Library of Congress)
Germany has also demonstrated adoption of PDTs through DiGA. Although not perfect, DiGA has established a revenue pathway that allows for DTx expansion and growth. There are over 60 products covered in Germany (DiGA-Verzeichnis). Germany is demonstrating the viability of the DTx promise: we can build products fast, bring them to market faster than pharmaceuticals, and adapt them faster. That promise was just a dream, until now.
We forget that biologics found their foothold in Europe well before they were adopted in the U.S. Do we also forget how many biologics companies went out of business before the industry took off? Here is a List of bankrupt biotech, pharma & medical device companies in Europe. For products that aren’t FDA regulated, or fall under enforcement discretion, think about the issues with supplements making claims: Label Claims for Conventional Foods and Dietary Supplements | FDA.
- Let’s gain perspective
The (DTx) industry is still young, and the initial excitement felt the real pains of healthcare. But now is THE TIME to pick ourselves up and take this industry to the next level as various initiatives and programs are aligning to drive real change. With CMS’s recent RFI in the Physician Fee Schedule and the increasing utilization of remote therapeutic and remote physiologic monitoring (RTM & RPM), alongside legislative pressures like the Access to Prescription Digital Therapeutics Act, policymakers are compelled to respond. Moreover, the FDA’s breakthrough and TAP programs, alongside PDURS, signify evolving market opportunities and exert ongoing pressure on CMS and commercial payors.
VC, pharma, payors, manufacturers and policymakers are looking for people to be realistic and grounded. We’re entering the slope of enlightenment in the Gartner Hype Cycle…Embrace it. (Gartner Hype Cycle Research Methodology)
And last but not least, Fierce Healthcare showed $2.7 Billion across 133 deals in Q1 2024, so we’re still seeing major investments in digital Q1 2024 digital health funding: Great (reset) expectations. With many DTx products already incorporating AI capabilities, I want to point out that AI interventions are DTx. While there is a new hype around AI, every roadblock to coverage of SaMD and DTx exists for AI interventions. DTA’s work on Capitol Hill applies to all software as a medical device delivering clinical validated therapeutics interventions
So let’s focus on what is working and look towards the next phase of DTx. Let’s rally around the success stories and use the pressure that we have put on policymakers to pave our way for success.
- Success Stories
Business models: Let’s look at what is working. 6 years ago this landscape didn’t exist. But the collective WE made THIS happen:
PDT in the EU: Board member Hannes Klöpper, CEO of HelloBetter, has 6 prescription products on DiGA. I also sat down for dinner in April with other DiGA companies. Their rate of bringing new products to market because of DiGA is that of the promises we heard in 2018 in the US. The phrase “We can bring products to market much faster than drugs” was heard around the clock in 2018, and Germany is showing that that is truly possible. France is following suit (watch the French Ministry of Health’s overview of PeCAN here)
Question: Would you consider launching in Germany and France first based on actual reimbursement models for PDTs?
DME: I can’t tell you how many times I have heard “DME will be the end of us”. Companies and associations were asserting to CMS that software as a medical device should be recognized as a medical device (imagine a drug company having to assert that a drug is indeed a drug). However, CMS’s stance was clear: they don’t reimburse for software alone. Interestingly, a company that initially sought parallel review was denied eligibility on the basis that CMS doesn’t reimburse for software. Yet, this same company is now classified as a medical device, demonstrating the importance of understanding and navigating CMS regulations in securing reimbursement.
Our most public example of a product getting DME classification is Applied VR. They now have a code from CMS and a reimbursement rate. Let’s strike through the statement that “DME will be the end of us”, because there is a big difference between coverage under DME or a reimbursement rate of $0. If this is the right pathway for your product then, just like PDTs in Germany, you have a viable business model and are able to demonstrate revenue and RWE to scale.
Chronic Care Management and SaaS models: “Care at home” is the next buzzy topic with policymakers as we look at our aging population. Well it isn’t a new topic for DTx companies. These platform models have been around for almost a decade now and have shown to be embraced by patients, particularly in diabetes. These models absolutely work. We have seen them transition into care platforms that include telehealth, coaching, medicine titration, etc. These have shown up now in diabetes, COPD, at home pulmonary rehab, and many more.
Pharma Partnerships: Pharma is starting to show enticing business models for DTx. This includes a therapeutic offering to build market awareness instead of an “unbranded campaign”, a lift to utilization, extensions to branded products, chronic care management to offer a higher touch approach to patient care, and the ability to have the sales force carry more products in their “bag”.
These models find stability due to recent PDURS regulatory considerations, signaling a future where prescription drugs, alongside DTx, can play a more significant role in patient care.
Provider Reimbursement: JOGO is demonstrating success by being paid as a provider. (Watch the JOGO Webinar here) We see RTM companies doing this to accompany their DTx, and we see software diagnostics companies like Digital Diagnostics using this model.
Last year, the idea that DTx could be reimbursed under the “incident to” benefit category started gaining traction. DTA submitted an RFI to CMS so that we could gain reimbursement via supply codes when providers’ offices procure the digital products. We have collectively put pressure on CMS to move reimbursement out of legislation. The next Physician Fee Schedule will be open for comment in early July. Regardless of what happens, you will see DTA continue to put pressure on CMS to move this reimbursement pathway forward.
Medicaid: In 2022, Massachusetts covered Reset and Reset-O in a value based agreement, Florida covered those products on the pharmacy benefit, and Oklahoma covered the product through a bulk purchase agreement. These are all opportunities for you if Medicaid is your market. IF you can show cost savings, you can also gain coverage in Managed Medicaid programs via “in lieu of services” Brief Leveraging In Lieu of Services in Medicaid Managed Care. You can also gain coverage through EPSDT programs. DTA put forth legislation in 2022 to help move this forward. If you want to see this move again, please contact us S.5238 – Medicaid and CHIP Access to Prescription Digital Therapeutics Act 117th Congress (2021-2022) .
Employer: There are many success stories in the employer space, but BE CAREFUL WITH EMPLOYERS! They are not a fail safe when other business models aren’t working. They have a very specific value prop. And people that work in HR benefits departments are not doctors looking at clinical evidence. BigHealth, for example, offers a very clear value proposition to employers: if you don’t sleep at night you won’t be productive during the day (my words, not the words of the BigHealth marketing department). Employers look at benefits around weight management like weight watchers and Noom, general health and wellness like gym memberships, family care, and areas that focus on productivity and employee retention. Landing these contracts are then followed by massive marketing campaigns to ensure that employees use your product. Employers are a great option if you have the right product.
PDTs in the US: OK, so we still have a major gap in reimbursement. If you don’t qualify as DME, then you will be told that SaMD isn’t covered by Medicare. Wait, but isn’t RPM and RTM covered by Medicare? Aren’t some services covered under the “incident to” benefit category? And weren’t we all told that it was impossible to get a HCPCS code if there is no benefit category right before the creation of A9291? (CMS officially separated coding from benefit categories and it turns out that the best way to get an answer from CMS is to do the obvious and just apply).
The PDT bill is stuck in the Congressional Budget Office because nobody knows how much the bill will cost, but the PDT bill has created an incredible amount of positive momentum for the industry regardless of its current status. While many got caught up in the details and worried about the harm of the PDT bill, this bill gave congress and policymakers a platform to drive education around DTx, point out issues with our healthcare system, and to put pressure on CMS. This pressure on CMS is compounded by programs in the FDA like the breakthrough program and TAP and policy like TCET. These programs are demonstrating a massive gap in Medicare coverage for innovative technologies.
And in the middle of all of this federal work, Highmark puts out a medical policy covering PDTs. highmark commercial medical policy – pennsylvania
PDTs are covered by a wide variety of purchasers, including the Veterans Administration, at risk health systems, and commercial payors. Scale in the US isn’t there yet, but we are certainly seeing advancements across the spectrum of payors.
FSA/HSA: And last but not least I guess: yes, DTx do qualify for the FSA/HSA. You just need to apply. Are you an OTC product? Great…allow your patients to pay using pre-tax dollars or company match programs.
These models and partnerships are exciting to VC, Pharma, Employers, at risk health systems, and purchasers in general. Let’s work together to drive these forward and change healthcare.
For next steps, come to our conference to hear DTA’s key initiatives and where you’ll get to talk to the FDA, the head of CPT, commercial payors, the VA, VC, pharma, and more: DTA Summit
And join our mission to make DTx available to all patients: Engage – Digital Therapeutics Alliance
Non-Prescription Digital Therapeutics: A Manufacturer and Employer Perspective
In the rapidly evolving landscape of healthcare, digital therapeutics (DTx) have emerged as a promising solution for improving patient outcomes and augmenting traditional medical interventions. In the recent webinar, Non-Prescription DTx Integration Workflows, the Digital Therapeutics Alliance (DTA) welcomed member companies, Sanofi and Woebot Health, along with an employer representative from WalkMe, to discuss how stakeholders come together to offer non-prescription DTx to employees.
Understanding Digital Therapeutics
Digital therapeutics are health softwares intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has a demonstrable positive therapeutic impact on a patient’s health. Digital therapeutics have the potential to benefit employees by providing accessible treatment for a wide variety of conditions.
Bringing Digital Therapeutics to Life: Workflows and Distribution Models
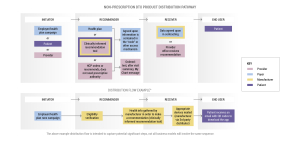
 Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Distribution Models for Non-Prescription Products
 The distribution of non-prescription digital therapeutics involves multiple stakeholders, including initiators, recommenders, receivers, and end-users. Initiators (health plans, employers, patients, or providers) play a pivotal role in recommending and facilitating access to these products.
The distribution of non-prescription digital therapeutics involves multiple stakeholders, including initiators, recommenders, receivers, and end-users. Initiators (health plans, employers, patients, or providers) play a pivotal role in recommending and facilitating access to these products.
Recommenders (health plans) employ various methods, such as text message campaigns or including digital therapeutics recommendations in after-visit summaries, to provide access to patients. Receivers (the provider) must ensure data exchange agreements are in place to safeguard patient privacy and security.
Ultimately, the end-user (the patient) stands to benefit from the accessibility and quality offered by non-prescription digital therapeutics.
Employer Perspective: A Case Study
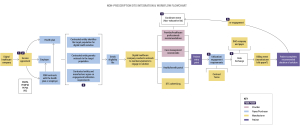
Let’s delve into a real-world scenario to understand how employers play a crucial role in facilitating the adoption of DTx and other digital health technologies (DHTs). Consider Jeremy, an employer representative, tasked with identifying and implementing digital solutions to address the health needs of his organization’s employees.
Jeremy’s journey begins with identifying the specific health needs of employees through direct outreach and engagement. Once the needs are identified, Jeremy collaborates with a broker to vet solutions that align with these needs. Demos and presentations from manufacturers play a pivotal role in the decision-making process, providing valuable insights into the functionality and efficacy of the products.
Multiple teams within the organization, including finance, information security, and legal, are involved in evaluating and implementing solutions to close gaps in healthcare delivery and improving outcomes for their employees. Understanding the needs and priorities of employers, such as data exchange, privacy, and security, is crucial during the contracting process.
Key Performance Indicators (KPIs) should be established early on to measure engagement and outcomes. This enables both parties to track progress and make data-driven decisions to optimize the effectiveness of digital therapeutics solutions.
Successful implementation of DTx requires a collaborative effort between manufacturers and employers with roles and responsibilities clearly mapped out. Manufacturers must provide clear and compelling promotional materials, including demos, to showcase the value of their products.
Conclusion
Digital therapeutics represent a paradigm shift in healthcare delivery, offering innovative solutions to address the evolving needs of patients and employers alike. By understanding the workflows and distribution models involved, manufacturers and employers can collaborate effectively to drive the adoption and utilization of digital therapeutics, ultimately improving patient outcomes and transforming the future of healthcare.
Learn more about DTA’s 2024 DTx Integration and Workflow Report.
 Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.