Value of DTx / Patients & Caregivers
New Effective Therapy Options
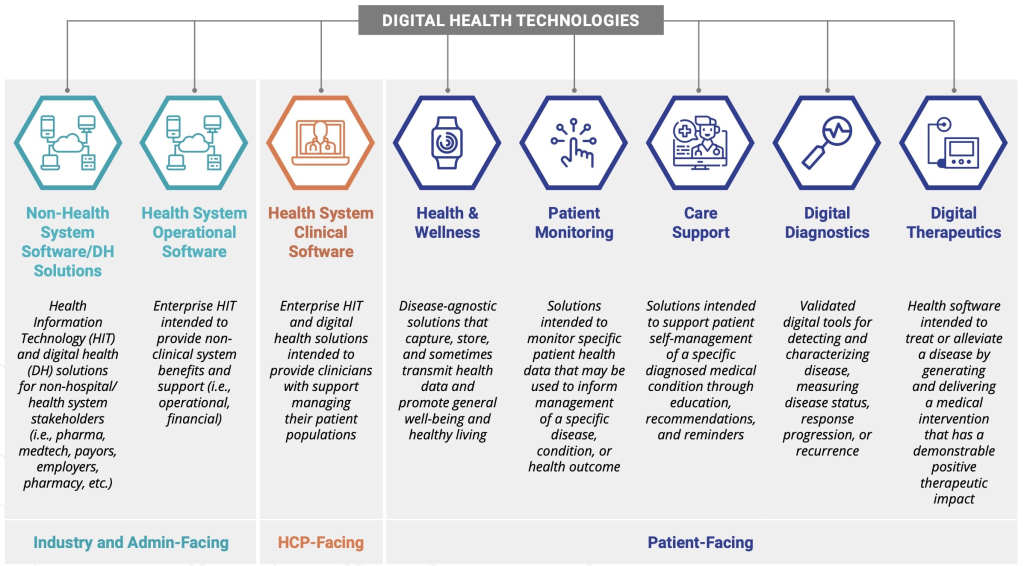
Digital therapeutics (DTx) are health softwares intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has a demonstrable positive therapeutic impact on a patient’s health.
DTx products deliver medical interventions directly to patients or integrate with other digital health technologies, medications, or in-person therapies to:
- Improve patient health
- Deliver a highly engaging and user-friendly experience
- Provide meaningful results and insights on personalized goals and outcomes
- Easily scale and be accessible via smartphones and tablets
- Ensure patient safety and privacy protection