Understanding DTx
A New Category of Medicine
Digital therapeutics (DTx) deliver medical interventions directly to patients using evidence-based, clinically evaluated software to treat, manage, and prevent a broad spectrum of diseases and disorders.

Digital therapeutics (DTx) deliver medical interventions directly to patients using evidence-based, clinically evaluated software to treat, manage, and prevent a broad spectrum of diseases and disorders.

DTx products are held to the same standards of evidence and regulatory oversight as traditional medical treatments.
Digital therapeutics should adhere to all core principles to demonstrate product safety, efficacy, quality, patient centricity, privacy, and ongoing clinical impact.
Digital therapeutics are essential to healthcare delivery systems. They eliminate gaps in care by:
DTx products can address critical gaps in care for underserved populations, regardless of patient age, language, culture, income, disease state, or geography. Read DTA’s report on the value of digital therapeutics for rural and underserved populations.

Product Library – To help key stakeholders understand and differentiate digital therapeutics from the thousands of other mobile health apps that are available, DTA developed this library to highlight evidence-based innovative DTx products.
Product LibraryDTx by Country – To answer common questions about these products, DTA developed country-specific resources to educate patients, clinicians, policymakers, and payors about DTx products and their value across global regions.
DTx by CountryThe products developed by DTA member companies address a wide variety of diseases and deliver an equally diverse array of interventions.
While DTA does not endorse, certify, or accredit products, we are glad to provide examples of DTx products from member companies that attest to aligning with DTx Industry Core Principles.
Visit DTA’s product library.
Digital therapeutic products must be proven effective via rigorous testing through Randomized Control Trials (RCT), ongoing real-world evidence generation, and analysis of product performance data. Clinical trial results are published in peer-reviewed journals to demonstrate that each technology:
Additionally, DTx products align with industry best practices which include well-established Good Clinical Practice protocols.
Digital therapeutics are regulated by national and regional agencies. DTx products adhere to similar standards of oversight as traditional medicines, which include:
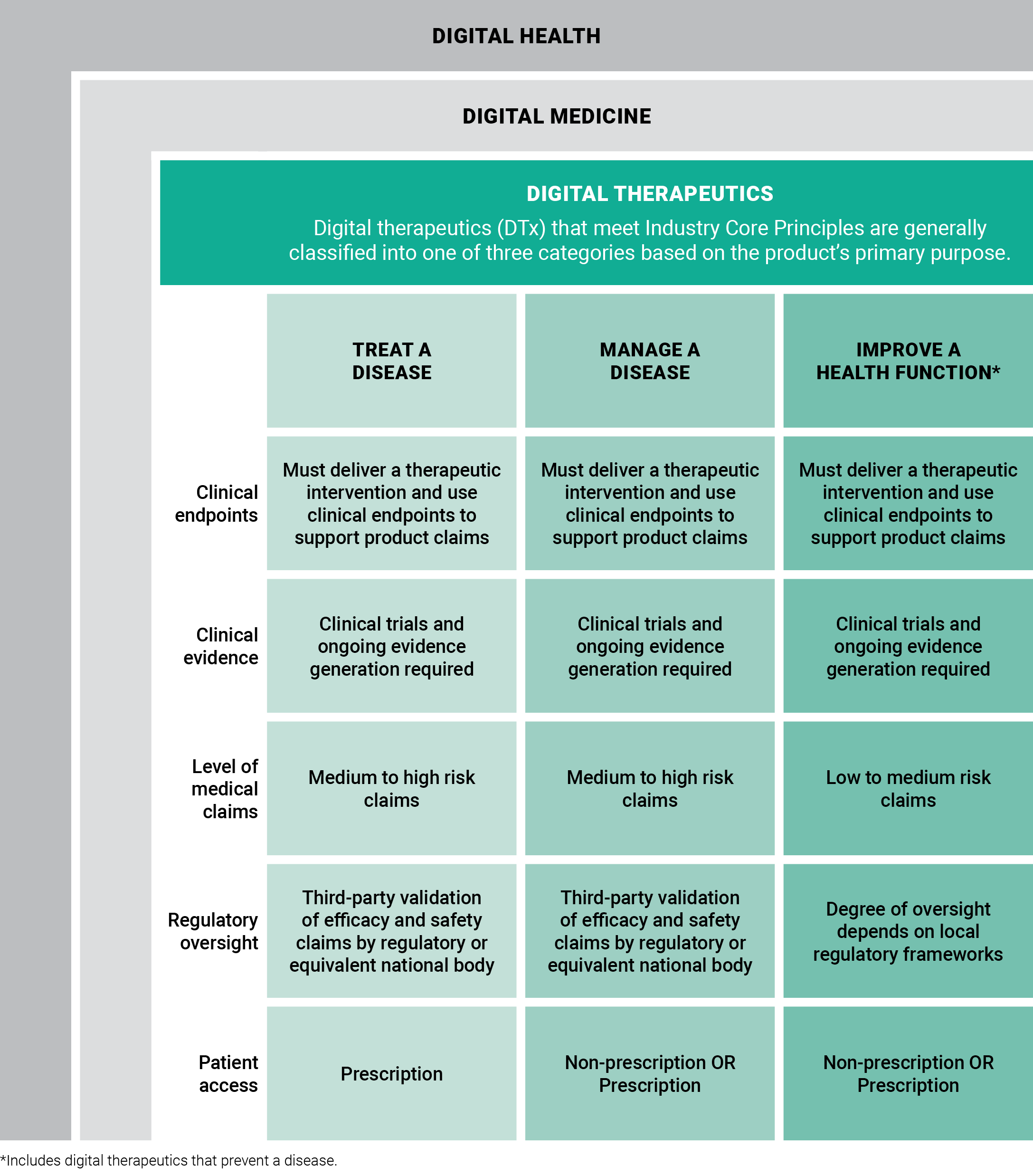
Even though all digital therapeutics adhere to industry core principles, these products fit into three different categories based on the product’s primary purpose and type of intervention delivered to patients. While exceptions may exist, DTx products generally align with one of these three categories: treat, manage, or prevent a disease (which includes the direct improvement of a health function).
Digital therapeutics improve patient outcomes for a wide range of diseases and disorders with clinically proven technologies accessible through devices such as smartphones or personal tablets.
DTx products may expand access to proven therapies, offer treatment options for un- or undertreated conditions, and empower patients with self-management tools. They are used independently or in concert with medications, devices, or other therapies to optimize patient care and health outcomes.
Additionally, real-world outcomes generated by digital therapeutics may be used to optimize outcomes at the individual patient and population levels. At the individual patient level, DTx products provide clinicians with meaningful, actionable clinical reports. At the population level, data generated by DTx products may be aggregated to track progress or compare aggregate outcomes based upon patient disease state, level of acuity, geographic location, age, gender, etc.
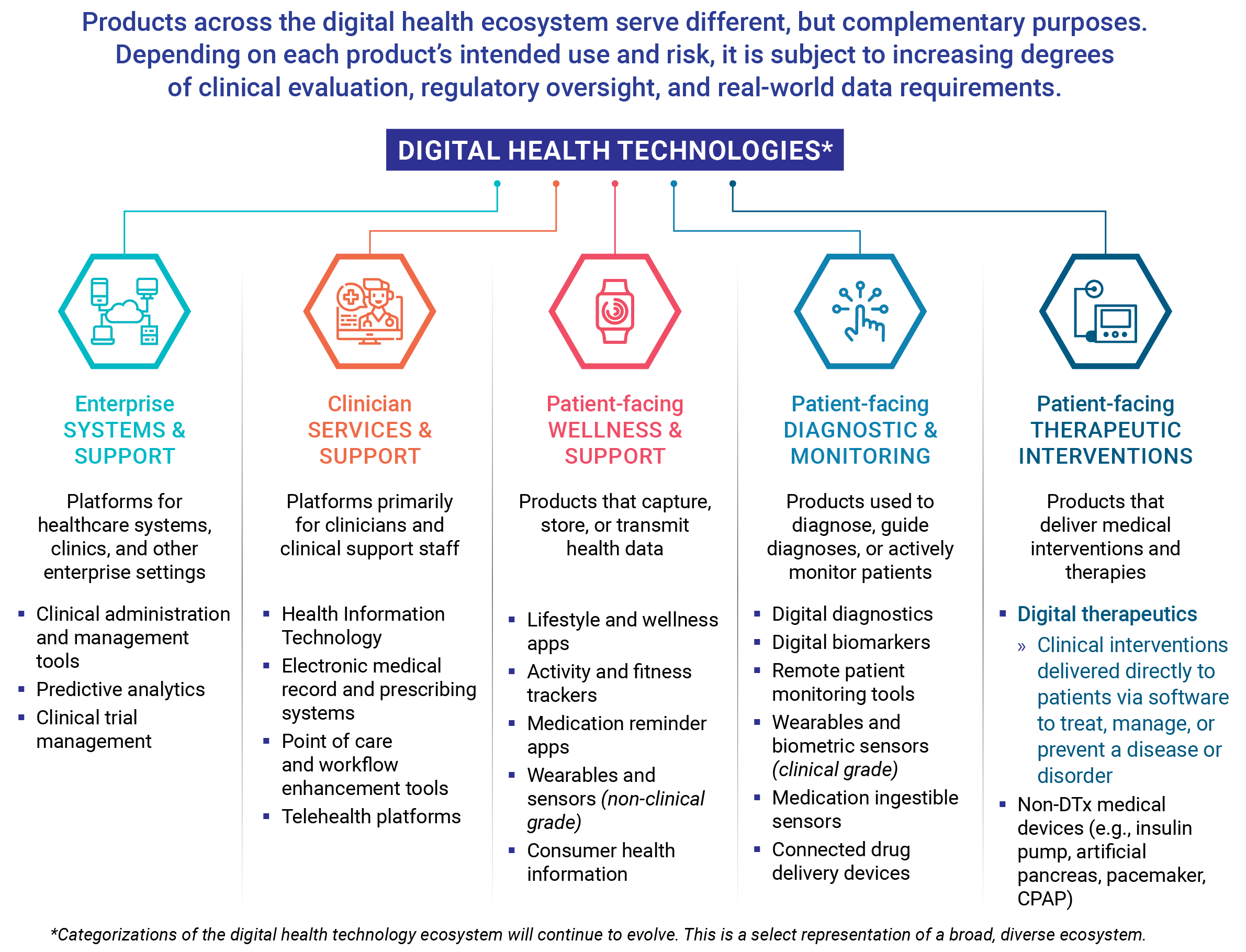
Compared to products that track fitness, encourage medication adherence, or monitor patient activity, DTx products deliver medical interventions directly to patients. As such, digital therapeutics undergo rigorous testing and are held to the same standards of evidence and regulatory requirements as traditional medical treatments.
Wellness and fitness apps that consumers download to their smartphones are not typically regulated, nor do they need to prove product claims by scientific testing or clinical evidence.
Patients & Caregivers – Gain access to proven technologies that are accessible, easy to use, and improve health outcomes.
Clinicians – Enhance the impact of your treatment plans to improve patient health and expand access to care.
Payors – Reduce overall medical costs, support value-based care initiatives, and improve member experience.