Product Highlight
Condition targeted:
Insonmina (Inc. chronic insomnia)


How it works:
Patients over the age of 18 years with insomnia (Inc. chronic insomnia).
Impact:
Improving a patient’s insomnia symptoms.
Product Overview
Medical condition:
Insomnia (Inc. Chronic Insomnia)
Target patient population:
Somzz is a type of digital therapeutics that can be applied to those over the age of 18 years to improve insomnia symptoms.
Somzz applies and implements the protocols of cognitive-behavioral therapy for insomnia (Stimulus Control Therapy, Sleep Restriction Therapy, Sleep Hygiene education, Relaxation Techniques, and Cognitive Therapy) through a mobile application.
This approach is considered the first-line recommended treatment for insomnia in clinical practice and Somzz provides education, real-time feedback, behavior intervention, and push notification message with a systematic algorithm to insomnia patients over 6 to 9 weeks.
What to expect:
More than half of the patients with chronic insomnia who participated in the clinical trial responded to “Somzz” with significant improvement in symptoms. In particular, 46.81% of the participants with insomnia symptoms showed remission at the end of the treatment program.
Clinical Overview
Indications for use:
Somzz is a Software as a Medical Device (SaMD) intended to improve insomnia symptoms by delivering a medical intervention (CBT-I) as a form of mobile application.
Outcomes:
The clinical trial demonstrated that more than half of the patients showed treatment response with significant improvement in symptoms using Somzz. In particular, 46.81% of the patients reached remission at the end of the treatment program.
Directions:
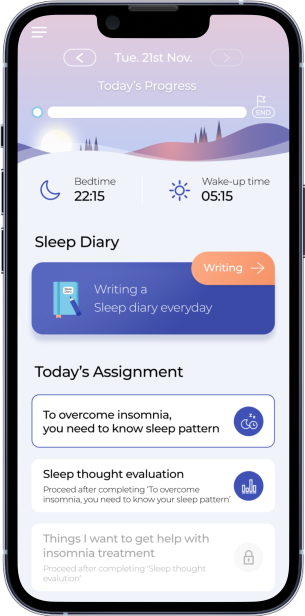
The patients used Somzz 2 to 3 times a day, for periods of 10–20 minutes. Somzz is used daily based on a planned and customized process for 6–9 weeks. The patients do daily assigned performances comprised of 6 treatment steps according to CBT-I, and a Sleep Diary completion followed by the recommended Time in Bed (TIB) based on the sleep restriction therapy.
- Stage 1: Basic education / sleep diary
- Stage 2: Stimulus control & Sleep restriction therapy / sleep diary
- Stage 3: Sleep hygiene education / sleep diary
- Stage 4: Relaxation therapy / sleep diary
- Stage 5: Cognitive therapy / sleep diary
- Stage 6: Relapse prevention education & Closing / sleep diary
Risks & warnings:
Somzz cannot be used if drowsiness due to sleep restrictions can cause serious accidents at the patient’s workplace or daily life. For patients with pathological conditions, it may also increase the risk for the pathophysiology to worsen. Thus, patients with negative conditions or the disabilities listed below cannot use Somzz as well: parasomnia, epilepsy, individuals at high risk of falling, individuals with other unstable or degenerative diseases.
Place in therapy:
Replaces face-to-face CBT-I therapy for insomnia.
Product Access
Product description:
Somzz is a Software as a Medical Device(SaMD) that applies and implements the protocols of cognitive-behavioral therapy for insomnia (CBT-I) through a mobile application with a systematic algorithm and provides education, real-time feedback, behavior intervention, and push notifications to insomnia patients over 6 to 9 weeks.
Prescription status:
A prescription from a qualified healthcare provider is required.
Patient access:
The patients get the APK link from medical staff and install Somzz on their Android phones or tablets. After, the patients need to sign up and proceed with the personal authentication process. Use of this product requires access to:
- Internet or wifi
- A mobile phone or tablet (Android only)
Provider access:
Healthcare providers may access the management web portal to prescribe procedures and monitor patients’ treatment progress through the generated health data.
Coverage options:
In progress – more details to come
Product availability:
Somzz is available in:
- South Korea: MFDS-Approved Class II Medical Device
Unique Features.
- Expanded Treatment Options: introducing a new treatment in addition to traditional medications and face-to-face CBT-I
- Remote Access: available via online access without physical constraints of space and time
- Real-time feedback: making it easy to track progress and engage patients actively in their treatment
- Safety: non-invasive and non-pharmacological, decreasing the safety issue
Clinical Trials
The provided set of evidence represents a sample of conducted studies. For a comprehensive collection contact manufacturers directly.
- Study Title: This publication is currently under review and will be posted shortly.
Study Design: Randomized controlled trial
Outcomes: In a clinical trial, the efficacy of Somzz for patients with chronic insomnia was proven superior to the placebo device, which consisted of a sleep diary and sleep hygiene education, demonstrating statistically significant improvement in insomnia severity scores at the end of treatment. The significant superiorities were also observed in the assessment scales such as Dysfunctional Beliefs and Attitudes about Sleep (dysfunctional beliefs), Patient Health Questionnaire-9 (depressive symptoms), Fatigue Severity Scale (fatigue), Epworth Sleepiness Scale (daytime sleepiness), 36-Item Short Form Survey (health-related quality of life) as well as in the sleep quality such as sleep efficiency and sleep onset latency. Regarding the remission and response rates, 46.81% of the patients randomized for Somzz presented with insomnia severity scores of 7 or under, and 51.06% had a decrease of 7 points or more at the end of the 6–9-week treatment.
Note: for more research, please visit