
This blog was written by Angela White, a patient advocate who attended the 2024 Digital Therapeutics Alliance Summit.
Exactly four weeks after discovering what I had assumed to be “digital therapeutics” (DTx) were just digital tools, I found myself entering the 2024 Digital Therapeutics Alliance Summit, preparing to offer insights as a panelist and patient advocate—despite my relatively nascent understanding. To say I felt overwhelmed and a bit intimidated would be accurate, so much so that I had written to make sure the organizers understood my lack of personal experience with DTx, despite my enthusiasm for potentially incorporating them into patient care across different conditions.
Immediately after learning about all that DTx includes, I did a deep dive into the topic, reviewing the Digital Therapeutics Alliance (DTA) website and searching academic journals— specifically those related to conditions with which I live. The DTA website proved illuminating. The academic journals proved scant. Yet, despite the dearth of DTx references in these journals, I realized that the field held great promise, particularly for complex, chronic, and progressive conditions. Living with multiple sclerosis (MS), I thought if I could see the potential for integrating DTx into the care relationship and communicate it effectively, then the lives of the more than 2.8 million people living with MS globally could improve.
Since many of the changes that occur with MS are subtle, occurring at a pace and level that are often not detectable by clinical examination, especially one that happens annually or biannually,
having a way to detect, assess, and mitigate these changes outside of clinic visits would be of immense benefit. They could also allow for more personalized treatment for this heterogeneous
group, as well as for many other patient populations that do not have a one-size-fits-all approach to treatment and care.
So, it was with this mindset that I attended and listened to presentations from various stakeholder groups:
As one who by nature is curious—and does not shy away from expressing my opinion—I left the Summit more committed to continuing to explore digital therapeutics and encouraging the
engagement of patient advocates as a central and necessary part of the digital therapeutics landscape. Why this commitment to centrality? It’s simple. Understanding patient populations, their needs, and what supports or hinders adoption and engagement with digital therapeutics (DTx) is essential. Without this insight, even the best intentions of industry developers may fall short in enhancing quality of life or effectively managing chronic conditions. While many developers are driven by both professional and personal motivations, these efforts must align with the real-world challenges patients face to truly succeed. Thankfully, DTA and their members recognize this and are inviting patients like me to be part of this collaborative engagement model. I’m already looking forward to the 2025 DTA Summit!
ABSTRACT
Digital health (DH) is driving a paradigm shift in health care that is transforming the way in which health services are delivered and received. DH applications are creating significant opportunities for health care stakeholders to improve patient outcomes, reduce unnecessary costs, and create additional value. To reap the benefits, multiple organizations, including pharmaceutical companies, hospitals, public and private payers, patient advocacy groups, traditional technology companies, and health technology companies, among others, have sought to develop and incorporate DH tools. Although DH has great potential value in oncology, the adoption of DH solutions in this area has been relatively low compared with other disease areas, such as diabetes and cardiovascular disease. This report outlines definitions of four promising categories of DH solutions in oncology and presents in-depth analyses of the current state of each category of solution. By piecing together the fragmented oncology DH market, the key drivers of successful DH solutions are illuminated, along with the actions needed to more effectively realize their future impact and value within oncology.
Digital therapeutics (DTx) have the ability to fill gaps in care for people and their families across the world. As a new category of medicine, one of the first barriers is the integration into the traditional healthcare system so that patients can receive access in a way that is convenient and consistent for them. This effort takes significant collaboration amongst diverse parties across the complex healthcare system in the United States.
These parties include policymakers from governing bodies, clinician and provider systems, health plans, pharmacies, DTx product manufacturers, and patients. However, there are many healthcare partners working behind the scenes to make it all work including pricing compendias and EHR vendors. Over the last two years, DTA has worked with these stakeholders in close partnership with NCPDP to address identified pain points in the workflows.
A critical barrier identified is understanding the payment pathways as they impact each stakeholder downstream. Through the generous support of the NCPDP Foundation, DTA was awarded a grant to research how DTx products would be reimbursed. Following the reimbursement workflows, we were able to finish the outline by addressing the distribution of the products into the hands of patients and their families.
We are proud of the efforts of our member task groups and other engaged stakeholders as we present this fourth and final DTx Integration & Workflow Report. While there are recommendations to improve the aspects of the healthcare system, we have been able to achieve our goal to outline how to integrate digital therapeutic products into the standard practice of care.
View final report (September 2024): DTx Integration and Workflow Report

Headlines
DTx in the News
After completing this activity, the participant should be better able to:
Over 650,000+ clinicians have used PIM for quality medical education and will now have access to DTx education!

As the healthcare industry undergoes tremendous transformation, this session will explore the evolving health information privacy and security landscape. Join us to learn about key changes like the final FTC health information breach rule and recent state law changes like Colorado’s new changes protecting “neural data” and their implications for digital health companies.
Objectives:
 LSX USA Congress gathers the founders and CEOs of innovative start-ups through to publicly listed healthcare giants, and everyone in between. It represents the breadth and depth of cutting-edge research and technology driving the advances in the industry right now and in the near future. Let us know if you’re attending!
LSX USA Congress gathers the founders and CEOs of innovative start-ups through to publicly listed healthcare giants, and everyone in between. It represents the breadth and depth of cutting-edge research and technology driving the advances in the industry right now and in the near future. Let us know if you’re attending!
Use code DTA15 to receive a 15% discount on registration.
Can’t attend at this time? Register to receive the recording post-event.
 Frontiers Healthis one of the premier health innovation events, with a strong focus on digital therapeutics, breakthrough technologies, healthcare transformation, investments, and ecosystem development. Our event brings together thought leaders, policy makers, industry players and innovative companies to dive deep into the ongoing challenges, strategic trends and scalable solutions likely to impact healthcare. Let us know if you’re attending!
Frontiers Healthis one of the premier health innovation events, with a strong focus on digital therapeutics, breakthrough technologies, healthcare transformation, investments, and ecosystem development. Our event brings together thought leaders, policy makers, industry players and innovative companies to dive deep into the ongoing challenges, strategic trends and scalable solutions likely to impact healthcare. Let us know if you’re attending!
DTA is proud to present a foundational Continuing Medical Education course designed to educate healthcare professionals about the rapidly evolving field of digital therapeutics. This CME program is designed to provide healthcare professionals with the knowledge and skills necessary to understand, implement, and optimize digital therapeutics in clinical practice.
This course, “Understanding the Future of Digital Therapeutics”, covers a wide range of topics to ensure thorough comprehension of digital therapeutics:
Access the CME course for free.
In the ever-evolving landscape of healthcare, where technology intersects with pharmaceuticals, the collaboration between digital therapeutics (DTx) companies and pharmaceutical companies is a testament to the potential synergies and challenges inherent in such partnerships. When DarioHealth and Sanofi US entered into a multi-year, $30m strategic agreement to help accelerate commercial adoption and expansion of the Dario platform, both organizations had much to learn. Their goals were to co-promote to health plans, develop new or enhanced solutions and generate robust evidence to support commercialization. This alignment and partnership shed light on the intricate dance between these two entities, showcasing the value they bring to each other and the lessons learned along the way.
Unveiling Mutual Value
At the heart of the partnership lies the recognition of the complementary strengths of both parties. For Dario, a digital therapeutics company, the allure of partnering with a pharmaceutical company like Sanofi is evident. As a smaller entity, Dario acknowledges the increased resources and capabilities that pharma brings to the table. From evidence generation to market research and commercialization, pharma’s expertise and access to sophisticated data open up new avenues for Dario to explore.
Conversely, pharma sees digital therapeutics as a means to augment traditional drug therapies, addressing gaps in patient care and enhancing treatment outcomes. With digital solutions offering personalized interventions and focusing on holistic patient management, pharma recognizes the value in diversifying treatment approaches beyond conventional medications.
Navigating Challenges
Beneath the surface of this partnership lie challenges that require delicate navigation. One such challenge is the stark contrast in operational workflows between pharma and digital therapeutics companies. While pharma is accustomed to the structured processes of drug development, DTx operates within a more iterative framework, especially in software and hardware development. Bridging this gap demands a deep understanding of each other’s operational intricacies.
Moreover, aligning the evidence generation strategy with the unique needs of both parties poses another hurdle. While Dario seeks to demonstrate the efficacy of its digital solutions in real-world settings, pharma aims to integrate these solutions seamlessly into existing treatment paradigms. Balancing these objectives requires meticulous planning and a tailored approach to evidence generation.
Building Trust and Collaboration
Central to overcoming these challenges is the cultivation of trust and collaboration from the outset. Building relationships founded on transparency and open communication fosters an environment where both parties can address concerns and adapt to evolving needs. By acknowledging the differences in expertise and operational models, partners can leverage their respective strengths to drive mutual success.
Key Takeaways
Reflecting on the journey of the Dario-Sanofi partnership, several key takeaways emerge:
Conclusion
The partnership between Dario and Sanofi exemplifies the potential for synergy and innovation when digital therapeutics and pharma converge. By navigating challenges with humility and a shared commitment to success, these partnerships have the power to revolutionize patient care and drive meaningful outcomes in healthcare. As the boundaries between technology and traditional medicine blur, embracing collaboration and embracing collaboration becomes essential in shaping the future of healthcare delivery.
In the rapidly evolving landscape of healthcare, digital therapeutics (DTx) have emerged as a promising solution for improving patient outcomes and augmenting traditional medical interventions. In the recent webinar, Non-Prescription DTx Integration Workflows, the Digital Therapeutics Alliance (DTA) welcomed member companies, Sanofi and Woebot Health, along with an employer representative from WalkMe, to discuss how stakeholders come together to offer non-prescription DTx to employees.
Understanding Digital Therapeutics
Digital therapeutics are health softwares intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has a demonstrable positive therapeutic impact on a patient’s health. Digital therapeutics have the potential to benefit employees by providing accessible treatment for a wide variety of conditions.
Bringing Digital Therapeutics to Life: Workflows and Distribution Models
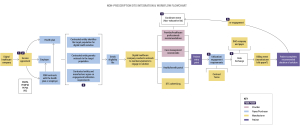 Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Distribution Models for Non-Prescription Products
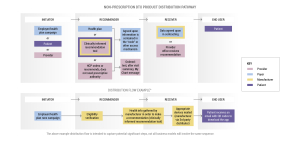 The distribution of non-prescription digital therapeutics involves multiple stakeholders, including initiators, recommenders, receivers, and end-users. Initiators (health plans, employers, patients, or providers) play a pivotal role in recommending and facilitating access to these products.
The distribution of non-prescription digital therapeutics involves multiple stakeholders, including initiators, recommenders, receivers, and end-users. Initiators (health plans, employers, patients, or providers) play a pivotal role in recommending and facilitating access to these products.
Recommenders (health plans) employ various methods, such as text message campaigns or including digital therapeutics recommendations in after-visit summaries, to provide access to patients. Receivers (the provider) must ensure data exchange agreements are in place to safeguard patient privacy and security.
Ultimately, the end-user (the patient) stands to benefit from the accessibility and quality offered by non-prescription digital therapeutics.
Employer Perspective: A Case Study
Let’s delve into a real-world scenario to understand how employers play a crucial role in facilitating the adoption of DTx and other digital health technologies (DHTs). Consider Jeremy, an employer representative, tasked with identifying and implementing digital solutions to address the health needs of his organization’s employees.
Jeremy’s journey begins with identifying the specific health needs of employees through direct outreach and engagement. Once the needs are identified, Jeremy collaborates with a broker to vet solutions that align with these needs. Demos and presentations from manufacturers play a pivotal role in the decision-making process, providing valuable insights into the functionality and efficacy of the products.
Multiple teams within the organization, including finance, information security, and legal, are involved in evaluating and implementing solutions to close gaps in healthcare delivery and improving outcomes for their employees. Understanding the needs and priorities of employers, such as data exchange, privacy, and security, is crucial during the contracting process.
Key Performance Indicators (KPIs) should be established early on to measure engagement and outcomes. This enables both parties to track progress and make data-driven decisions to optimize the effectiveness of digital therapeutics solutions.
Successful implementation of DTx requires a collaborative effort between manufacturers and employers with roles and responsibilities clearly mapped out. Manufacturers must provide clear and compelling promotional materials, including demos, to showcase the value of their products.
Conclusion
Digital therapeutics represent a paradigm shift in healthcare delivery, offering innovative solutions to address the evolving needs of patients and employers alike. By understanding the workflows and distribution models involved, manufacturers and employers can collaborate effectively to drive the adoption and utilization of digital therapeutics, ultimately improving patient outcomes and transforming the future of healthcare.
Learn more about DTA’s 2024 DTx Integration and Workflow Report.
In the ever-evolving landscape of healthcare innovation, we often witness the rise and
fall of pioneering companies. Today, we acknowledge that Better Therapeutics has
decided to wind down operations and delist from the Nasdaq. While the company’s
journey has come to an end, it’s crucial to recognize and appreciate their contributions
to the DTx industry and support the longevity of their products for people with type 2
diabetes.
The bankruptcy of Better serves as a sobering reminder of the inherent risks associated
with healthcare startups. The road to success in this industry is fraught with challenges,
including regulatory hurdles, funding uncertainties, market adoption barriers, and clinical
validation requirements. Leaving many people wondering, where did Better go wrong?
We are witnessing a systemic problem within the U.S. healthcare system. Patient
access to digital health technologies is a crucial aspect of modern healthcare, yet CMS
and private health plans fail to provide coverage for these FDA cleared products. While
countries like Germany, France, UK, South Korea, and Japan have implemented
initiatives to facilitate patient access to digital therapeutics, the U.S. lags behind. It is
disheartening that in a country as advanced as the United States, our governing bodies,
agencies, and representatives will not take the steps to ensure that patients receive the
care they need. This is yet another stark example of the shortcomings of the U.S.
healthcare system and the bureaucracy that plagues it, leaving patients at a
disadvantage.
Digital health represents the future of healthcare. With advancements in technology,
data analytics, and artificial intelligence, digital solutions have the potential to
revolutionize patient care, improve outcomes, and enhance the efficiency of healthcare
delivery. From remote patient monitoring and personalized interventions to virtual care
platforms and predictive analytics, the possibilities are endless.
The story of Better Therapeutics is not an industry signal – there are many examples of
financial successes in digital health. Let us express our gratitude for their contributions
and commit ourselves to advancing the field of digital therapeutics with renewed
determination and optimism. With perseverance, collaboration, and a shared vision for
the future of healthcare, we can overcome challenges and realize the full potential of
digital health to transform lives
Digital therapeutics (DTx) have potential to fill gaps in care for people and their families across the world. As a new category of medicine, one of the first barriers is the challenge of integration into the traditional healthcare system so patients can receive access in a way that is convenient and consistent for them. This effort takes significant collaboration amongst diverse stakeholders across the complex healthcare system in the United States.
In January 2023, DTA released the DTx Integration & Workflow Report to identify gaps and pain points in integrating digital therapeutic products into workflows across the U.S. healthcare system and outline necessary next steps to optimize the patient and clinician experience. The report identified pain points, considerations to solve for, and next steps in solving for those pain points. DTA continued to work with collaborators across the healthcare ecosystem and with NCPDP Task Groups to publish a Progress Report in August 2023.
A critical barrier identified is understanding the payment pathways as it impacts each stakeholder downstream. Through the generous support of the NCPDP Foundation, DTA was awarded a grant to research how DTx products would be reimbursed. The research is included in this updated report. We are proud of the efforts of our member task groups and other engaged stakeholders as we present this third DTx Integration & Workflow Report.
View full report (March 2024): DTx Integration & Workflow Report