Astellas and Welldoc Enter into Strategic Alliance for Digital Therapeutics
Astellas Pharma Inc. and Welldoc, Inc. today announced that the companies have entered into a collaboration and license agreement directed toward the development and commercialization of digital health solutions. Under the agreement, Astellas and Welldoc will jointly develop and commercialize BlueStar® in Japan and certain other Asian markets for patients with diabetes, collaborate to broaden the adoption of BlueStar® in the U.S. market, and jointly develop and commercialize digital therapeutics in other therapeutic areas globally.
Propeller Health users can now access pharmacy services from CVS, Walmart, Kroger and Rite Aid from within the Propeller app
Propeller Health, a leading digital health company dedicated to the management of asthma and chronic obstructive pulmonary disease (COPD), announced that users are now able to access pharmacy services from CVS, Walmart, Kroger and Rite Aid directly from the Propeller app.
In January 2019, Propeller announced the launch of My Pharmacy, an in-app feature that allows users to manage their prescription refills for asthma and COPD and locate a nearby pharmacy from the Propeller app.
DarioHealth® Clinical Study Data Highlights the Material Benefit Digital Interventions Have on Glycemic Control in Users with Diabetes
DarioHealth Corp. (Nasdaq: DRIO) (“DarioHealth” or “Dario”), a leading global, digital therapeutics company, presented new clinical data yesterday at the 19th Annual Diabetes Technology Meeting (“DTM”) conference in Bethesda, Maryland, which showed remarkable improvements in average blood glucose levels (BGavg) and in-range measurements in patients with diabetes using Dario’s digital therapeutics platform and dedicated one-on-one personal health coaching.
DarioHealth’s digital therapeutics platform delivers personalized, evidence-based interventions to users that are driven by precision data analytics, high quality software and one-on-one coaching, which are all available through Dario’s suite of membership plans. The clinical study data presented at the DTM conference included users enrolled in Dario’s full membership plan, which is available to payers, providers and consumers.
Blue Cross and Blue Shield of Minnesota Launches Omada’s Type 2 Diabetes Management Program
Deepening a collaboration that began in 2013, Omada Health and Blue Cross and Blue Shield of Minnesota (Blue Cross) today announced the availability of Omada’s Type 2 Diabetes Digital Care program as a covered benefit for the insurer’s commercial plans. Building on the two organizations’ success deploying digital diabetes prevention in the state, Omada’s type 2 diabetes program will be available for Minnesota employers to launch beginning January 2020. Blue Cross also announced a strategic investment in Omada Health through the insurer’s parent company.
Digital Therapeutic Industry Leaders Promote Ethical Standards and Product Development Best Practices
Trust is crucial as patients increasingly use digital therapeutic products for the delivery of high quality, evidence-based medical treatments
Washington, D.C. — Today, the Digital Therapeutics Alliance (DTA) released an Industry Code of Ethics, a series of Best Practices, and an updated product claims categorization chart for digital therapeutic (DTx) products. DTA is also proud to collaborate this week with the Digital Medicine Society, HealthXL, and NODE.Health to release a broad categorization of the Digital Health, Digital Medicine, and Digital Therapeutic industries.
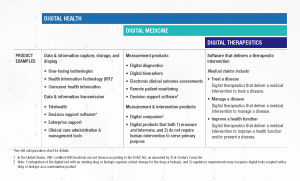
DTx products deliver personal and adaptive medical interventions to patients and are driven by high quality, evidence-based software programs. As such, they make claims to prevent, manage, or treat a medical disease, and therefore carry a higher level of risk than other products in the broader digital health landscape. Digital therapeutics do not include lifestyle and wellness apps, clinical decision support, or products that deliver static, automatic interventions to patients.
“DTx products provide a monumental opportunity to improve how healthcare is delivered by increasing therapeutic options for unmet medical needs and expanding patient access and engagement. Given the importance of these therapies, DTA members appreciate the responsibility of protecting patients and end users every step of the way,” shares Megan Coder, DTA Executive Director. “In many ways, digital therapeutics are an extension of a clinician in a patient’s everyday life as they receive active treatment outside of a clinic. This is why these documents are so important.”
In order to reinforce the foundations of the digital therapeutic industry, DTA is proud to release the following:
- DTx Code of Ethics: As the DTx industry continues to expand and deliver medical interventions directly to patients, it is important for end users to know that the companies working to develop, evaluate, and market digital therapeutics are acting in the best interest of patients. Therefore, DTA member companies collaborated with fellow leaders in the healthcare industry to develop a DTx Industry Code of Ethics. This Code demonstrates DTA Member Companies’ dedication to developing and bringing DTx products to market in a responsible way.
- Industry Best Practices: Last year, DTA published Industry Core Principles to which all DTx products should adhere. These principles address topics such as robust product quality and design, privacy and security, end user engagement, and clinical evaluation. In order to provide better clarity for patients, clinicians, and payors on how to assess product alignment with these principles, DTA compiled a series of Best Practices that products may utilize to demonstrate robust design, evaluation, and delivery of interventions.
- DTx Product Claims: In 2018, DTA published an Industry Report that provided the definition of a DTx product, industry principles, and a chart representing DTx product claim types. In order to provide a more appropriate representation of the industry, DTA recently developed a more streamlined approach to the DTx Product Claims categorization.
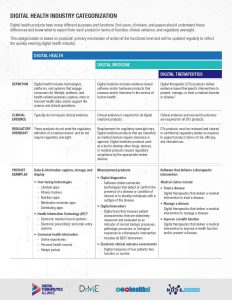
- Industry Categorization: Clarity and trust are crucially important in this quickly evolving industry. With this mission, DTA collaborated with other organizations to outline the landscape of digital health products available to end users and clinicians. This Digital Health Industry Categorization demonstrates how products making higher-risk medical claims must undergo greater levels of clinical evidence and regulatory oversight.
“Currently there is tremendous confusion among patients, clinicians, and payors about the types of products that exist across the broader digital health landscape,” said Coder. “It is important for patients and other users to understand what types of digital products they are using, what products’ primary functions are, and the levels of clinical outcomes and regulatory oversight that are required of them.”
These resources demonstrate that not all digital products are the same, and those products that are delivering medical interventions and making medical claims are taking a serious approach to ensuring patient safety, protection, and care.
Access these materials on https://dtxalliance.org/aboutdtx/.
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding and adoption of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payers in order to improve clinical and health economic outcomes. To learn more please visit: www.dtxalliance.org.
Updated: 13 November 2019
Digital Health, Digital Medicine, Digital Therapeutics (DTx): What’s the difference?
Clarity matters. Here’s what you need to know.
Digital health products have become integral to the prevention, diagnosis, treatment, and management of health and disease. Consumers rely on digital health apps to improve their focus, track their fitness, and optimize their wellbeing. Clinicians use digital health products to gain insights on patient outcomes, conduct telehealth visits, and treat aspects of diseases otherwise unaddressed by traditional medications.
In theory, consumers and clinicians know that there is a difference between these products. But in practice, how do they differentiate between products that accurately collect health information versus those that do not, or those that provide clinicians with informational versus actionable insights?
Not all digital health products are the same. But what are their differences? How should each product be used? What should expectations be about product outcomes? How much evidence is needed to go to market? What type of clinical evidence or regulatory oversight is necessary?
NICE’s evidence standards framework for digital health technologies and the eHSG (European Commission eHealth Stakeholder Group) Guidance, both released this year, address varying requirements within the digital health universe. With the industry garnering such support, the need to categorize and differentiate between its various solutions becomes even more important.
Providing clarity and trust is crucial in this quickly evolving industry. With this as our mission, the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health are collaborating to describe the landscape of digital health products available to end users and clinicians, in addition to the level of clinical evidence and regulatory oversight that correlates to each product category.
As these transformative technologies are laying the groundwork for a new form of healthcare, we must ensure that the digital tools we are placing our trust in are indeed worthy of that trust.
Product risk matters
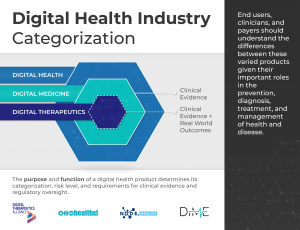
Digital Health is a broad category that encompasses Digital Medicine, which in turn includes Digital Therapeutics. Products in these categories make different levels of claims and therefore have different levels of risk. As such, they have varying requirements for clinical evidence and regulatory oversight.
In parallel industries such as transportation, higher levels of research and regulation are required for higher risk functions (e.g., operating autonomous vehicles) than lower risk functions (e.g., bluetooth enabled hands-free car kits). Similarly, end users and clinicians should expect that higher levels of evidence and oversight are required for digital health products that make higher risk claims.
Thus, the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health have collaborated to propose this risk-based framework so end users may discern between products of different risk and corresponding levels of necessary evidence and regulatory oversight.

Why categorize the digital health industry?
Rock Health, the first venture fund dedicated to digital health, defines digital health as the space at the intersection of technology and healthcare. HealthXL shares this broad perspective and serves the entire and extensive field of digital health through its market intelligence platform and expert community.
DiMe focuses on digital medicine, the field of evidence-based digital health tools that measure and/or intervene in the service of health to support the practice of medicine broadly, including treatment, recovery, disease prevention, and health promotion for individuals and across populations.
NODE.Health is a membership-based organization with a mission to bring the rigor of evidence-based science to digital medicine. NODE.Health builds on and explores the knowledge base now required to lead global healthcare systems and industry into the value-based era of digital transformation in health.
DTA is the industry association advancing the field of digital therapeutics (DTx). DTx products employ high quality software to deliver evidence-based therapeutic interventions that prevent, manage, or treat a broad spectrum of physical, mental, and behavioral conditions.
These distinctions are intended to clarify, not complicate, the field of digital health. We agree with our FDA and NIH colleagues that:
Effective, unambiguous communication is essential for efficient translation of promising scientific discoveries into approved medical products. Unclear definitions and inconsistent use of key terms can hinder the evaluation and interpretation of scientific evidence and may pose significant obstacles to medical product development programs.
We define these categories to support:
- Consumers of digital health tools — health systems, clinicians, researchers, payers, and patients — seeking to select the most fit-for-purpose digital products for their needs.
- Developers and manufacturers of digital health tools in pursuing the most appropriate go-to-market strategies for their products.
Specifics are invaluable
The digital health field is not yet served by a standard lexicon, and the many disciplines that comprise digital health are often separated by a common language. Anyone who has ever been part of a conversation about ‘validation’, for example, knows that every discipline has its own definition.
This categorization framework goes beyond the existing definitions of digital health, digital medicine, and digital therapeutics to provide clarity with examples of products and product types.

The digital health universe is rapidly expanding — both in size and capabilities. Although this categorization is not intended to be wholly comprehensive, DiMe, DTA, HealthXL, and NODE.Health commit to regular framework updates to ensure it evolves with the industry and remains a valuable tool for all stakeholders.
How to use this framework
This framework is not intended to assign hierarchy to the different digital health product categories. No category is more or less valuable to the advancement of health, healthcare, and/or health research; they are simply governed by different evidence and regulatory requirements.


In addition, users of this framework should not assume that digital health products have no degree of risk compared to digital medicine products or digital therapeutics. While the latter two are subject to higher evidence requirements and regulatory oversight due to greater clinical risk, commercial digital health tools may pose their own unique risks.
For example, many digital health products may boast robust evidence despite its not being required, and the absence of such requirements can make it challenging to identify ‘digital health’ apps that have been proven effective. Similarly, there may be unintended consequences associated with the use of digital products without a robust body of evidence attesting to their safety, efficacy, and equality of performance.
Digital tools falling into the digital health category are currently governed by caveat emptor, or “buyer beware”. This is particularly challenging in an industry plagued by information asymmetry.
Further, privacy and security concerns transcend this framework’s categorical boundaries of digital health, digital medicine, and digital therapeutics. Even when the necessary clinical evidence is available for a digital health product, other considerations such as security features, ethical approaches to data rights and governance, and economic feasibility should be considered.
Why now?
There is a pressing need for further work to better characterize digital health products based on holistic assessments of risk.This is necessary to support trust in digital health, digital medicine, and digital therapeutics, and from there, realize their potential to improve health, healthcare, and health research.
By using this categorization framework, we trust that: patients will better understand what digital products they are using and for what purpose, clinicians will better leverage digital health products in practice and know how they relate to other treatments, payers will better understand the landscape of products available to patients and the type of value and clinical outcomes they should expect, and pharma will see the R&D requirements and commercial opportunities each offers.
Wellness products deliver different outcomes than diagnostic products. Adherence products require different types of evidence and regulation than therapeutic products. Clinical decision support tools support clinicians differently than telehealth products.
Clarity matters. And together our organizations are glad to be part of this conversation.
Readers: We value your feedback, and encourage you to submit your comments on the framework here.
________
Authors: Jennifer Goldsack, Megan Coder, Chandana Fitzgerald, Natalie Navar-Mattingly, Andy Coravos, and Ashish Atreja, MD
________
The Digital Medicine Society (DiMe) is a Massachusetts non-profit and professional society for individuals from all backgrounds working to advance digital medicine to optimize human health.
The Digital Therapeutics Alliance (DTA) is a non-profit trade association with the mission of broadening the understanding and integration of clinically-evaluated digital therapeutics into healthcare to improve clinical and health economic outcomes.
HealthXL is the market intelligence platform and expert community for digital health.
NODE.Health is a 501(c)(3) non-profit organization dedicated to education, validation and dissemination of evidence based digital medicine.
PKG Wristwatch Device May Improve Clinical Decision-Making in Parkinson’s, Study Shows
A wrist-worn medical device — called the Personal KinetiGraph or PKG — that reminds people with Parkinson’s disease to take their medication, and records their movements to provide physicians with objective measurements of motor symptoms, showed great promise as a tool to improve clinical decision-making, a study found.
When worn for six days before a routine care visit, the device reported disease-related motor symptoms, like dyskinesia — involuntary muscle movement — and bradykinesia, or the progressive slowness of movement over time, even when patients did not report such symptoms.
Pear Therapeutics Announces Agreement with Ironwood Pharmaceuticals to Evaluate Prescription Digital Therapeutics for Patients with GI Indications
Pear Therapeutics, Inc. announced today that it has entered into an agreement with Ironwood Pharmaceuticals, Inc. to evaluate Prescription Digital Therapeutics (PDTs) for the treatment of selected indications within the gastrointestinal (GI) space.
This collaboration will leverage Pear’s platform and capabilities in PDTs with Ironwood’s innovative GI franchise expertise.
“We are excited to be partnering with Ironwood on this initiative and believe there is a compelling clinical rationale for use of PDTs in treating GI diseases,” said Corey McCann, M.D., Ph.D., President and CEO of Pear Therapeutics. “This represents an important step for Pear and broadens our pipeline to diseases outside the central nervous system and into a range of chronic conditions.”
What if we get things right? Visions for 2030
World Economic Forum (Ceri Parker) — We asked members of our Global Future Councils – academics, business leaders and members of civil society – to imagine a better world in 2030. Only by thinking about where we want to be tomorrow can we prompt the action we need today. Here’s what they had to say…
Propeller users can now locate their lost inhaler by “ringing” their Propeller sensor from the app
Starting today, Propeller users will be able to “ring” the sensor on a misplaced inhaler to make it easier to find, through an update to the Find My Inhaler feature in the Propeller app.
Propeller is a digital health tool that helps people with asthma or COPD manage their breathing. Propeller sensors connect to patients’ existing inhalers and transmit data on medication use and health patterns to the Propeller app on patients’ smartphones. This is the latest update from Propeller aimed at making life better for people with asthma or COPD, based on feedback from patients about the day-to-day realities of their condition.