Navigating the Intersection of Pharma and Digital Therapeutics: A Blueprint for Successful Partnerships
In the ever-evolving landscape of healthcare, where technology intersects with pharmaceuticals, the collaboration between digital therapeutics (DTx) companies and pharmaceutical companies is a testament to the potential synergies and challenges inherent in such partnerships. When DarioHealth and Sanofi US entered into a multi-year, $30m strategic agreement to help accelerate commercial adoption and expansion of the Dario platform, both organizations had much to learn. Their goals were to co-promote to health plans, develop new or enhanced solutions and generate robust evidence to support commercialization. This alignment and partnership shed light on the intricate dance between these two entities, showcasing the value they bring to each other and the lessons learned along the way.
Unveiling Mutual Value
At the heart of the partnership lies the recognition of the complementary strengths of both parties. For Dario, a digital therapeutics company, the allure of partnering with a pharmaceutical company like Sanofi is evident. As a smaller entity, Dario acknowledges the increased resources and capabilities that pharma brings to the table. From evidence generation to market research and commercialization, pharma’s expertise and access to sophisticated data open up new avenues for Dario to explore.
Conversely, pharma sees digital therapeutics as a means to augment traditional drug therapies, addressing gaps in patient care and enhancing treatment outcomes. With digital solutions offering personalized interventions and focusing on holistic patient management, pharma recognizes the value in diversifying treatment approaches beyond conventional medications.
Navigating Challenges
Beneath the surface of this partnership lie challenges that require delicate navigation. One such challenge is the stark contrast in operational workflows between pharma and digital therapeutics companies. While pharma is accustomed to the structured processes of drug development, DTx operates within a more iterative framework, especially in software and hardware development. Bridging this gap demands a deep understanding of each other’s operational intricacies.
Moreover, aligning the evidence generation strategy with the unique needs of both parties poses another hurdle. While Dario seeks to demonstrate the efficacy of its digital solutions in real-world settings, pharma aims to integrate these solutions seamlessly into existing treatment paradigms. Balancing these objectives requires meticulous planning and a tailored approach to evidence generation.
Building Trust and Collaboration
Central to overcoming these challenges is the cultivation of trust and collaboration from the outset. Building relationships founded on transparency and open communication fosters an environment where both parties can address concerns and adapt to evolving needs. By acknowledging the differences in expertise and operational models, partners can leverage their respective strengths to drive mutual success.
Key Takeaways
Reflecting on the journey of the Dario-Sanofi partnership, several key takeaways emerge:
- Open-mindedness: Embrace the potential for growth and innovation by remaining receptive to new ideas and possibilities, even if they challenge preconceived notions.
- Humility: Check egos at the door and recognize that collaboration is a two-way street. Valuing the perspectives and expertise of both parties fosters a culture of mutual respect and understanding.
- Alignment of Business Models: Ensure that the evidence generation strategy aligns with the broader business objectives of both parties. Tailoring the approach to meet the needs of key stakeholders enhances the relevance and impact of the generated evidence.
- Iterative Learning: Approach partnerships as opportunities for continuous learning and improvement. Embrace the iterative nature of the collaboration process, allowing for course corrections and adaptations along the way.
Conclusion
The partnership between Dario and Sanofi exemplifies the potential for synergy and innovation when digital therapeutics and pharma converge. By navigating challenges with humility and a shared commitment to success, these partnerships have the power to revolutionize patient care and drive meaningful outcomes in healthcare. As the boundaries between technology and traditional medicine blur, embracing collaboration and embracing collaboration becomes essential in shaping the future of healthcare delivery.
Non-Prescription Digital Therapeutics: A Manufacturer and Employer Perspective
In the rapidly evolving landscape of healthcare, digital therapeutics (DTx) have emerged as a promising solution for improving patient outcomes and augmenting traditional medical interventions. In the recent webinar, Non-Prescription DTx Integration Workflows, the Digital Therapeutics Alliance (DTA) welcomed member companies, Sanofi and Woebot Health, along with an employer representative from WalkMe, to discuss how stakeholders come together to offer non-prescription DTx to employees.
Understanding Digital Therapeutics
Digital therapeutics are health softwares intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has a demonstrable positive therapeutic impact on a patient’s health. Digital therapeutics have the potential to benefit employees by providing accessible treatment for a wide variety of conditions.
Bringing Digital Therapeutics to Life: Workflows and Distribution Models
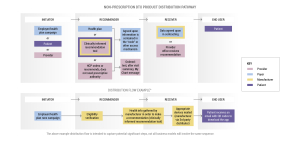
 Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Distribution Models for Non-Prescription Products
 The distribution of non-prescription digital therapeutics involves multiple stakeholders, including initiators, recommenders, receivers, and end-users. Initiators (health plans, employers, patients, or providers) play a pivotal role in recommending and facilitating access to these products.
The distribution of non-prescription digital therapeutics involves multiple stakeholders, including initiators, recommenders, receivers, and end-users. Initiators (health plans, employers, patients, or providers) play a pivotal role in recommending and facilitating access to these products.
Recommenders (health plans) employ various methods, such as text message campaigns or including digital therapeutics recommendations in after-visit summaries, to provide access to patients. Receivers (the provider) must ensure data exchange agreements are in place to safeguard patient privacy and security.
Ultimately, the end-user (the patient) stands to benefit from the accessibility and quality offered by non-prescription digital therapeutics.
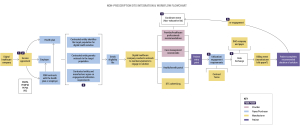
Employer Perspective: A Case Study
Let’s delve into a real-world scenario to understand how employers play a crucial role in facilitating the adoption of DTx and other digital health technologies (DHTs). Consider Jeremy, an employer representative, tasked with identifying and implementing digital solutions to address the health needs of his organization’s employees.
Jeremy’s journey begins with identifying the specific health needs of employees through direct outreach and engagement. Once the needs are identified, Jeremy collaborates with a broker to vet solutions that align with these needs. Demos and presentations from manufacturers play a pivotal role in the decision-making process, providing valuable insights into the functionality and efficacy of the products.
Multiple teams within the organization, including finance, information security, and legal, are involved in evaluating and implementing solutions to close gaps in healthcare delivery and improving outcomes for their employees. Understanding the needs and priorities of employers, such as data exchange, privacy, and security, is crucial during the contracting process.
Key Performance Indicators (KPIs) should be established early on to measure engagement and outcomes. This enables both parties to track progress and make data-driven decisions to optimize the effectiveness of digital therapeutics solutions.
Successful implementation of DTx requires a collaborative effort between manufacturers and employers with roles and responsibilities clearly mapped out. Manufacturers must provide clear and compelling promotional materials, including demos, to showcase the value of their products.
Conclusion
Digital therapeutics represent a paradigm shift in healthcare delivery, offering innovative solutions to address the evolving needs of patients and employers alike. By understanding the workflows and distribution models involved, manufacturers and employers can collaborate effectively to drive the adoption and utilization of digital therapeutics, ultimately improving patient outcomes and transforming the future of healthcare.
Learn more about DTA’s 2024 DTx Integration and Workflow Report.
 Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.
Bringing digital therapeutics into the healthcare ecosystem involves navigating complex workflows and distribution models. From the manufacturer’s perspective, it requires a comprehensive understanding of the needs of both patients and employers, as well as collaboration with various stakeholders to ensure successful adoption.