Product Highlight
Condition targeted:
Post-traumatic stress disorder (PTSD), panic disorder, panic/anxiety attacks

How it works:
Freespira is an FDA-cleared at-home treatment that addresses the symptoms associated with panic disorder, panic/anxiety attacks, and PTSD. With its unique mechanism of action, Freespira corrects dysfunctional breathing associated with CO2 hypersensitivity by training patients to stabilize their breathing. By combining hardware/software with personal coaching, Freespira has demonstrated its ability to reduce or eliminate panic attacks and PTSD symptoms in just 28 days.
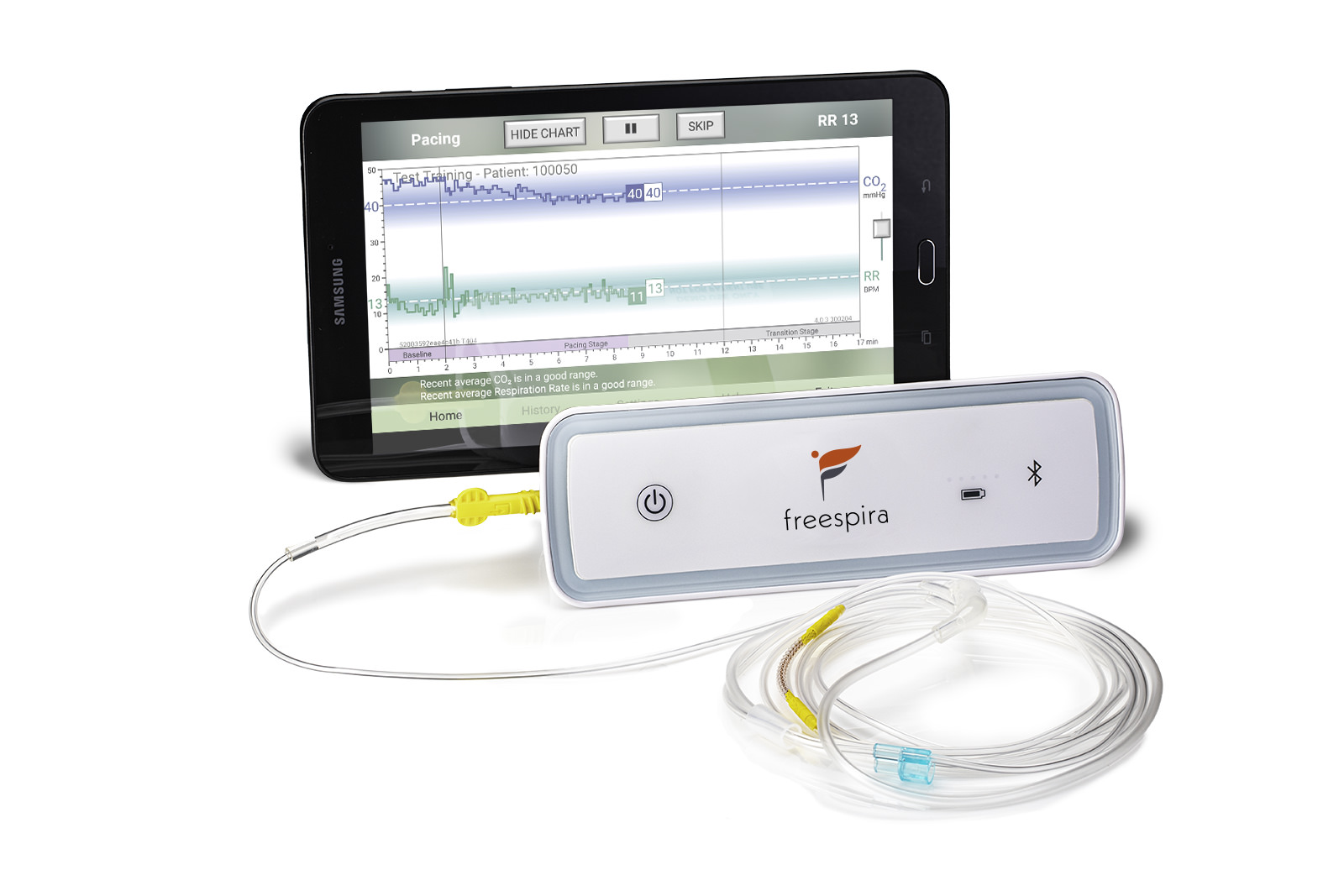
The at-home Freespira system includes:
- A compact breathing sensor which samples the patient’s breathing through a nasal cannula
- A tablet that provides real-time feedback of the breathing pattern for the patient to act on
Impact:
Freespira has delivered consistent clinical outcomes across 6 published studies. In clinical trials focused on panic disorder, ≥80% of patients treated reported significant reduction or elimination of symptoms post treatment. More impressively, Freespira also had an enduring impact with a majority of patients maintaining clinical response through 12-months. In a clinical trial focused on PTSD, 89% of patients reported a significant decrease in symptoms based on CAPS-5 Score and 50% of patients were in remission at 6 months.
Freespira also has published health economic outcome (HEOR) data that provided significant reductions across key costs, including: medical costs by 35%, emergency department by 65%, and pharmacy costs by 68%.
Sources: Kaplan, et al., 2020; Ostacher, et al., 2021
Product Overview
Medical conditions:
Freespira has two FDA indications: Post-traumatic stress disorder (PTSD) and Panic Disorder (which includes panic or anxiety attacks)
Target patient population:
Patients over 18 years old with PTSD, panic disorder, or suffering from panic attacks associated with other medical or behavioral health conditions.
What to expect:
Significant reduction or elimination of panic and/or PTSD symptoms
Note: for more information, please visit https://freespira.com/for-individuals/.
Clinical Overview
Indications for use:
Freespira is indicated as an adjunctive treatment for symptoms associated with panic disorder (PD) and/or post-traumatic stress disorder (PTSD), to be used under the direction of a healthcare professional, together with other pharmacological and/or non-pharmacological interventions
Outcomes:
- Clinical Outcomes
In real world data, clinical outcomes seen confirmed those achieved in clinical studies:
-
- 86% of panic disorder and panic attack patients were panic attack-free immediately post-treatment
- 73% of patients were panic attack-free at 12 months post-treatment
For PTSD patients, at 6-months post-treatment:
-
- 89% reported a significant decrease in symptoms based on the CAPS-5 Score (validated PTSD assessment scale)
- 50% were in remission
- Economic Outcomes
For health plan costs, one prospective study showed:
-
- 35% reduction in medical costs
- 65% reduction in emergency department costs
- 68% reduction in pharmacy costs
Sources: Kaplan, et al., 2020 and Ostacher, et al., 2021
Directions:
After receiving training by a clinician or Freespira coach, patients complete an at-home 28-day protocol with two 17-minute breathing sessions each day, for one month. Four weekly virtual coaching sessions are also included.
Risks & warnings:
Not indicated for patients who are younger than 13 years old, are pregnant, or have chronic obstructive pulmonary disease (COPD) or emphysema
Place in therapy:
Freespira is adjunctive to current treatments for PTSD and panic. However, neither psychotherapy nor medications are required for patients using Freespira.
Product Access
Product description:
Freespira corrects dysfunctional breathing associated with CO2 hypersensitivity. When people with CO2 hypersensitivity are exposed to everyday anxiety, it can trigger panic attacks. People with PTSD share this CO2 hypersensitivity and dysfunctional breathing, which sets the stage for post-traumatic flashbacks and other symptoms. By teaching patients who suffer from panic attacks and PTSD to normalize breathing using Freespira, everyday anxiety no longer triggers a catastrophic bodily response.
The Freespira treatment includes:
- Two 17-minute sessions per day for 28 days
- Training and weekly check-ins by secure video with a care coordinator/coach
The at-home Freespira system includes:
- A compact breathing sensor which samples the patient’s breathing through a nasal cannula
- A tablet that provides real-time feedback of the breathing pattern for the patient to act on
Prescription status:
Authorization from a licensed healthcare provider is required. A prescription from a physician is not necessary.
Patient access:
Health plans, self-insured employers and the Veterans Administration provide Freespira’s drug-free solution to improve quality of life, reduce medical expenditures and support the appropriate use of valuable healthcare resources.
Patients have two pathways for enrollment into the Freespira program: self-referral through the website, or referral from a healthcare provider. Once referred, patients will engage with a Freespira advisor to verify benefits and validate the patient meets clinical criteria for enrollment. This advisor will also explain the onboarding process to begin treatment.
Provider access:
Healthcare providers may refer a patient to Freespira via website, phone, or fax. Providers, including behavioral health, primary care, psychiatry, and case management can receive post-treatment reports for their patients. Veterans Administration healthcare providers receive additional clinician support via Freespira field teams and approved government contractors.
Coverage options:
Coverage examples include the Veterans Administration, Highmark Health (see Highmark medical policy here), and Children’s Community Health Plan, who offer Freespira as a covered benefit for eligible members with panic disorder, panic attacks, or PTSD symptoms. For patients who receive Freespira through employers, Comcast for example, there are no out-of-pocket costs associated with product use. Freespira is also offered as a self-pay product if insurance companies do not offer coverage.
Product availability:
Freespira is available in:
- USA: FDA-cleared Class II Medical Device
Unique Features
Freespira is the only FDA-cleared, evidence-based, medication-free digital therapeutic that can reduce or eliminate the symptoms of panic disorder, panic attacks, and PTSD by addressing the underlying physiologic factors related to these conditions.
Clinical Trials
The provided set of evidence represents a sample of conducted studies. For a comprehensive collection contact manufacturers directly.
- Study Title: Feedback of End-tidal pC02 as a Therapeutic Approach for Panic Disorder. Alicia E. Meuret, Ph.D., Frank H. Wilhelm, Ph.D., Thomas Ritz, Ph.D., and Walton T. Roth, M.D.
Study Design: Randomized Controlled Trial
Outcomes: 68% panic attack free 12 months post-treatment; 93% clinically significant reduction in panic symptoms 12 months post-treatment; and 91% treatment adherence - Study Title: A Multisite Benchmarking Trial of Capnometry Guided Respiratory Intervention for Panic Disorder in Naturalistic Treatment Settings. David F. Tolin, Patrick B. McGrath, Lisa R. Hale, Daniel N. Weiner, and Ralitza Gueorguieva
Study Design: Mutli-Center Study
Outcomes: 71% panic attack free immediately post-treatment; 79% panic attack free 12 months post-treatment; 82 % clinically significant reduction in panic symptoms 12 months post-treatment; and 84% treatment adherence - Study Title: Investigation of Investigation of a Capnometry Guided Respiratory Intervention in the Treatment of Posttraumatic Stress Disorder in the Treatment of PTSD. Michael J. Ostacher, Eileen Fischer, Ellie R. Bowen, Jihun Lyu, Denishia J. Robbins, and Trisha Suppes.
Study Design: Single Arm Trial
Outcomes: At 6-months post-treatment, 89% had significant reduction in CAPS-5 Score (validated PTSD assessment); 50% of patients were in remission; 77% treatment adherence - Study Title: Real-world Evaluation of a Novel Technology-enabled Capnometry-assisted Breathing Therapy for Panic Disorder. Divya K. Madhusudhan, Kore N. Glied, Eugene Nguyen, Jennifer Rose, and Dena M. Bravata.
Study Design: Employer-sponsored Onsite Clinic Program
Clinical Outcomes: Immediately post treatment, 67% of participants had a clinically significant (40% or greater) reduction in their PDSS score. Engagement was associated with decline in utilizations of behavioral health services at employer-sponsored primary care center clinic after the intervention - Study Title: Evaluating the Impact of Freespira on Panic Disorder Patients’ Health Outcomes and Healthcare Costs within the Allegheny Health Network. Alicia Kaplan, Anthony P. Mannarino, and P.V. Nickell
Study Design: Health Economic Study
Clinical Outcomes: 86% panic attack free immediately post-treatment; 73% panic attack free 12 months post-treatment; 94% clinically significant reduction in panic symptoms 12 months post-treatment; and 83% treatment adherence
Economic Outcomes: 35% reduction in overall medical costs; 65% reduction in emergency room costs; and 68% reduction in medication costs measured at 12 months post treatment
Note: for more research, please visit https://freespira.com/resources/.