The Digital Therapeutics Alliance Welcomes Introduction of Bipartisan Legislation to Provide Guidance to U.S. States on Public Coverage Options for Prescription Digital Therapeutics
The Digital Therapeutics Alliance Welcomes Introduction of Bipartisan Legislation to Provide Guidance to U.S. States on Public Coverage Options for Prescription Digital Therapeutics
The guidance related to prescription digital therapeutic coverage would help U.S. states leverage existing programs to expand DTx access for patients covered by Medicaid and State Children’s Health Insurance Program (CHIP)
ARLINGTON, VA – December 14, 2022 – As the leading international organization on digital therapeutic (DTx) thought leadership and education, the Digital Therapeutics Alliance (DTA) welcomes the introduction of the ‘‘Medicaid and CHIP Access to Prescription Digital Therapeutics Act’’. This bipartisan legislation would provide guidance regarding coverage of prescription digital therapeutics under Medicaid and the State Children’s Health Insurance Program (CHIP).
This bipartisan legislation is championed by Senators Shelly Moore Capito (R-WV) and Jeanne Shaheen (D-NH) and reflects the growing recognition of the clinical and health economic value that digital therapeutics provide to patients, caregivers, and clinicians – especially given the increased barriers to care that Medicaid beneficiaries continue to face during and beyond the Covid-19 pandemic.
While Massachusetts and Oklahoma are already covering prescription digital therapeutics through their state Medicaid programs, this legislation provides much-needed clarity for other states intending to provide access to digital therapeutics and comes at a critical moment for patients, when many are unable to access other forms of necessary therapies. Using this guidance to leverage existing pathways to expand access to DTx products for Medicaid-covered populations will enable those with chronic and mental health conditions to access expanded care options that can make a tremendous difference in their quality of life and healthcare outcomes.
In addition to providing critical guidance to enable more standardized coverage decisions across the country, the Medicaid and CHIP Access to Prescription Digital Therapeutics Act (S.5238) would also allow the Secretary of Health and Human Services (HHS) to provide technical assistance to states considering coverage of FDA-approved or cleared prescription digital therapeutic products, and would define ‘prescription digital therapeutic’ within the context of Medicaid.
“Digital therapeutics hold particular value for Medicaid populations with convenient, accessible, and personalized treatment options to address many unmet medical needs,” says Andy Molnar, DTA Chief Executive Officer. “This legislation would establish more clarity and uniformity in how prescription digital therapeutics are covered by public programs from state to state and is a critical step toward ensuring that these evidence-based treatments get into the hands of those who need them most. We are grateful for the work of the bill sponsors and look forward to working with a broad coalition of patients, clinicians, and others to pass this into law.”
###
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
Media Contact:
Hannah Fairman
Digital Therapeutics Alliance
hfairman@dtxalliance.org
Digital Therapeutics Alliance and Curebase release publication setting the stage for a fit-for-purpose evidence standard for digital therapeutics (DTx)
Digital Therapeutics Alliance and Curebase release publication setting the stage for a fit-for-purpose evidence standard for digital therapeutics (DTx)
Publication provides a foundational set of expectations outlining the necessary clinical evidence to enable appropriate and efficient assessment reflective of the unique nature and innovation cycles of DTx products
Arlington, VA and San Francisco, CA – Tuesday, December 6, 2022 – The Digital Therapeutics Alliance (DTA), a global non-profit trade association with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare, in collaboration with DTA Resource Partner, Curebase, a company committed to democratizing access to clinical studies, today released a publication to provide a fit-for-purpose evidence standard for DTx product regulatory, reimbursement, and clinical acceptance.
The publication, “Setting the Stage for a Fit-For-Purpose DTx Evidentiary Standard”, outlines foundational principles specific to the DTx category of medicine and baseline expectations for healthcare decision makers (HCDMs) related to the types, quality, and timing of clinical trials necessary to evaluate and implement DTx therapies in real-world settings.
Given the profound growth of the DTx industry over the last decade, and as more HCDMs across the world embark on evaluating DTx products, it is important for clinicians, policymakers, and payors to have access to a harmonized, consistent set of study expectations to sufficiently demonstrate product safety, efficacy, and impact. Thus far, DTx products have been subject to the same clinical evidence standards that are used to assess the safety and effectiveness of other traditional medical devices and pharmaceuticals. However, given the unique nature of DTx products, it is increasingly clear that a fit-for-purpose evidence evaluation standard is required to account for the faster and iterative nature of DTx product life cycles, levels of potential risk, and their place in clinical therapy.
“For broader patient access to DTx therapies across the global marketplace, evaluation frameworks need to be clear, consistent, and specific to the unique characteristics of DTx products,” said Megan Coder, Chief Policy Officer of the Digital Therapeutics Alliance. “A fit-for-purpose DTx product evidence evaluation framework – reflective of how DTx products are designed and used in real-world settings – strengthens DTx evidentiary robustness and clarity, thereby preventing unnecessary delays in patient access to high-quality clinically-validated digital therapeutics.”
“Patients everywhere deserve access to high-quality care; having a fit-for-purpose evidence standard for DTx brings the industry one step closer to this,” says Whitney Stewart, Director of Clinical Project Management at Curebase. “By aligning the industry and providing the foundation for DTx studies, this paper paves the way for more thorough DTx studies, for a clearer path forward for DTx companies, and should result in overall better patient care.”
This publication provides evidence-based expectations for how DTx products should be validated by payors globally, building on principles from pharmaceutical and general medical device standards, while acknowledging digital therapeutics’ unique mechanisms of action, iterative development lifecycles, and clinical outcomes. To enable movement toward a fit-for-purpose DTx evidentiary standard, DTA will continue to engage with the broader healthcare ecosystem to develop harmonized and DTx-specific clinical evidence frameworks. For more information, and to download the paper, visit: https://dtxalliance.org/wp-content/uploads/2022/12/DTA-Clinical-Evidence-Paper_12.22.pdf.
###
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors in order to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
About Curebase
At Curebase, our mission is to bring quality medical innovations to patients faster and improve human well-being through more efficient clinical studies. We are proving that clinical research can be radically accelerated if we empower physicians everywhere to enroll patients in the communities where they live. By applying cutting-edge clinical software and remote study management techniques to the problem, we are reinventing clinical trials and research from the ground up. For more information, please visit www.curebase.com.
DTA Welcomes Board of Directors for 2023
DTA Welcomes Board of Directors for 2023
DTA Board Members provide strategic vision, oversight, and guidance to the organization’s work to transform global healthcare by advancing digital therapeutics.
Tuesday, November 8, 2022 – Arlington, VA – Today, the Digital Therapeutics Alliance (DTA), is pleased to announce the results of the 2023 DTA Board of Directors election. With two seats up for election this cycle, DTA members selected Danny Kim, Head of WELT USA, as a new board member and re-elected Owen McCarthy, Co-Founder and President of MedRhythms, Inc. and current Chair to serve on the Board.
The 2023 DTA Board of Directors:
- Everett Crosland – Chief Commercial Officer, Cognito Therapeutics
- Anand Iyer – Chief Strategy Officer, Welldoc
- Danny Kim – Head of WELT USA
- Eddie Martucci – Co-Founder and CEO, Akili Interactive
- Owen McCarthy – President and Co-Founder, MedRhythms
- Debra Reisenthel – Founding CEO and Board Member, Freespira, Inc.
- Julia Strandberg – Chief Commercial Officer, Pear Therapeutics, Inc.
- Andy Molnar – Chief Executive Officer, Digital Therapeutics Alliance
The Board of Directors supports DTA’s work to transform global healthcare by advancing digital therapeutics (DTx) and provides critical guidance related to the strategy and direction of the organization.
“We are looking forward to working with this Board to capitalize on the organizational initiatives already underway and tackle additional innovative efforts to advance digital therapeutics,” said Andy Molnar, DTA’s Chief Executive Officer. “These industry leaders will draw on their experience building successful companies to provide crucial support guiding DTA’s work to expand patient access to DTx products to improve clinical and health economic outcomes.”
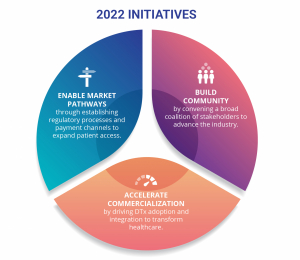
DTA’s Board has set the strategic vision to transform global healthcare by advancing digital therapeutics and their oversight and guidance will be instrumental in ensuring successful execution:
- Enable market pathways: Establish clear and consistent market pathways to ensure DTx product coverage and improved access.
- Accelerate commercialization: Drive DTx product development, adoption, and integration to transform healthcare.
- Optimize real-world use: Prioritize the patient and clinician experience in the development and delivery of safe, effective and equitable DTx products.
Debra Reisenthel, Vice-Chair of the DTA Board of Directors, shares, “Strong leadership from industry trailblazers has been essential to support the rapid evolution of the DTx industry as well as the considerable growth of the Alliance, which now represents over 100 companies across 20 countries. I am excited to work with my fellow directors to pursue our strategic focus in 2023 on advancing adoption and integration of digital therapeutics on a global scale.”
###
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors in order to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
DTA Advocates for Better Digital Therapeutics Access and Coverage
DTA Advocates for Better Digital Therapeutics Access and Coverage
DTx industry members held 50 meetings with congressional representatives to gain support for critical legislation at event in Washington, DC
 September 26, 2022 – Arlington, VA– This past week, the Digital Therapeutics Alliance (DTA) held a Capitol Hill Advocacy Event in Washington, DC for member companies and allied partners to discuss policy and legislative opportunities to provide better access and coverage for digital therapeutics (DTx) and gain support for H.R. 7051/ S. 3791 the “Access to Prescription Digital Therapeutics Act of 2022”.
September 26, 2022 – Arlington, VA– This past week, the Digital Therapeutics Alliance (DTA) held a Capitol Hill Advocacy Event in Washington, DC for member companies and allied partners to discuss policy and legislative opportunities to provide better access and coverage for digital therapeutics (DTx) and gain support for H.R. 7051/ S. 3791 the “Access to Prescription Digital Therapeutics Act of 2022”.
DTA staff and member organization participants met with representatives from both the U.S. House of Representatives and Senate leadership, including senior advisors from the offices of Speaker of the House Nancy Pelosi (D-CA) and Senate Minority Leader Mitch McConnell (R-KY) to discuss the value of digital therapeutics and the opportunities to improve clinical and economic outcomes with expanded access to DTx products. Advocating for expanded adoption, coverage, and access to clinically validated digital therapeutics for the benefit of patient care, DTA members also met with key committee representatives from Senate Finance, House Ways and Means, and House Energy and Commerce.
In total, over the course of the two-day event, DTx industry leaders met with staff from over 25 Senate and 25 House offices, representing 29 states, as well as the District of Columbia, to educate lawmakers on digital therapeutics and urge support for passage of the “Access to Prescription Digital Therapeutics Act”. This bipartisan legislation was introduced in March 2022 with five sponsors. DTA kicked off efforts to advance this important piece of legislation during an advocacy event in Washington, DC shortly after and since then, the bill has steadily gained support, with co-sponsors in the House and Senate now numbering more than 20. If passed, the legislation would establish benefit categories for a subset of digital therapeutics and would provide a much needed access pathway for the over 160 million people covered by Medicare, Medicaid, and other publicly-funded programs.
“The Access to Prescription Digital Therapeutics Act is a critical piece of legislation to provide equitable access to quality medical treatments,” said Andy Molnar, DTA CEO. “It’s been incredible to be a part of this community driving action on the Hill and I am proud of the collective work we have done to gain support for this much needed legislation.”
DTA is looking forward to continuing to meet with other Members of Congress over the coming weeks to secure more support for this legislation in addition to expanding other efforts to establish clearer pathways for DTx products to improve patient outcomes. Connect with us if you would like to be involved.
Special thanks go to event sponsors Otsuka Pharmaceutical and Eversana, and DTA Resource Partners, Foley Hoag and Susan Stoner Zook, Mason Street Consulting.
About Digital Therapeutics Alliance
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
Digital Therapeutics Alliance and Healthware Group Partner to Build European Coalition to Scale Access to Digital Therapeutics
Digital Therapeutics Alliance and Healthware Group Partner to Build European Coalition to Scale Access to Digital Therapeutics
Arlington, VA – Milan, Italy | 14 June 2022 – The Digital Therapeutics Alliance (DTA) and Healthware Group today announced a partnership to form a coalition convening thought leaders, policymakers, and professional and trade associations from across the European region to discuss and develop harmonised pathways for the recognition and scalability of digital therapeutics (DTx) at the local, national, and regional levels.
Developing this pan-European coalition will be a catalysing factor for the successful scaling of DTx therapies across countries. The first in-person meeting of the coalition will take place at Frontiers Health Global Conference this coming October in Milan, Italy. The coalition will focus on co-creating a vision for the future by analysing existing frameworks and infrastructure, sharing learnings, and developing recommendations to build an environment which ensures safe and effective DTx therapies can be accessible to patients regardless of where they live.
The partnership between Healthware and DTA is part of the Alliance’s Resource Partner program, a best-in-class network of leading experts in the rapidly evolving DTx industry who collaborate with DTA members to develop resources to advance digital therapeutics to transform global healthcare. Through this relationship, Healthware will also support DTA’s Europe Policy Task Group, which is focused on understanding and informing key aspects of the European policy landscape, including Health Technology Assessment frameworks, national funding, clinician engagement, and patient access and reimbursement pathways.
This announcement follows a longstanding collaboration between the two organisations, which began with DTA’s European launch at the Frontiers Health Global Conference in 2017. The development of a pan-European coalition will deepen DTA’s presence in the region and further its mission to broaden the understanding, adoption, and integration of clinically evaluated digital therapeutics into healthcare through education, advocacy, and cross-industry collaboration.
Healthware brings over a decade of experience and advocacy of digital health/digital therapeutics and an even longer history of developing digitally focused solutions for life sciences, payors and medical device companies. Healthware has also developed a corporate investment fund to support and invest in digital health companies and nurtures a vast digital health network and expertise. In the area of digital therapeutics specifically, Healthware Group supports both the research and development of DTx products as well as the go to market, access, system integration and adoption strategies in partnership with pharmaceutical companies, digital health and DTx companies and all ecosystem stakeholders.
“We have been partnering with Healthware since the inception of DTA and are thrilled to have their knowledge and expertise on board,” shared Megan Coder, DTA Chief Policy Officer. “As we jointly develop this coalition, our aim is to enable full scale access to digital therapeutics throughout Europe to transform critical aspects of patient care.”
Roberto Ascione, CEO of Healthware Group says:
“My team at Healthware has been helping shape the digital health landscape in Europe for many years, through our work with Frontiers Health and in direct support of our clients. We are excited to deepen our partnership with DTA to help shape the European policy landscape and ensure DTx companies can scale. We are huge advocates for the positive impact digital therapeutics can have on patients and believe that these solutions can help fill care gaps, augment care delivery and most importantly support patients in novel ways.”
Healthware and DTA look forward to building this coalition together and are seeking the active participation of key stakeholders across the region. Further announcements about the coalition and how to get involved will be rolling out soon.

About Digital Therapeutics Alliance
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
Contact:
Hannah Fairman, Director of Communications
Email: hfairman@dtxalliance.org
Healthware Group
Healthware Group is a global health innovation and technology leader providing transformational advisory and technology services for commercial, medical, and R&D operations of life-sciences and digital health companies, combined with design and development of digital medicines and digital therapeutics products.
Proprietary software platforms, specialized media and educational assets as well as a corporate venturing arm, ensure accelerated product development, close integration within the innovation ecosystem, continuous pipeline development and superior market access capabilities.
Founded in Italy in 1997 by CEO and digital health pioneer Roberto Ascione, Healthware Group encompasses several vertical brands, including flagship commercial and medical communications agency Healthware International, media consultancy Healthware Engage, innovation consultancy Healthware Labs, and creative motion lab & virtual hybrid events specialist SWM and the digital therapeutics R&D partner and product portfolio organization, Healthware Therapeutics.
It also operates Healthware Ventures, the corporate investment arm that supports digital health start-ups with a focus on digital therapeutics and telehealth and is the co-host of the global leading digital health conference Frontiers Health.
Healthware has a team of 200+ professionals with main offices in Salerno, London, New York, Milan, Rome, Helsinki and, together with its joint venture partner Eversana Intouch, has a combined reach of 2000+ people in over 15 offices in Europe, the US, and Asia.
Additionally, the Healthware Global Network, one of the largest international networks of independent healthcare agencies, provides for deep local expertise worldwide in over 25 countries.
Healthware Group is privately owned and backed by FITEC, a leading European VC fund focusing on technology.
For more information, please visit healthwaregroup.com and follow us on LinkedIn and Twitter.
Contacts
Antonietta Pannella, Marketing & Communications Director
Mobile: +39 349 0648276 – Email: antonietta.pannella@healthwaregroup.com
DTA’s recent webinar “Embarking on a new era for digital therapeutics- the DTx Value Assessment & Integration Guide”
Recording now available to DTA’s recent webinar “Embarking on a new era for digital therapeutics- the DTx Value Assessment & Integration Guide”
With digital therapeutics marking a decade of market availability, the DTx industry is demonstrating a strong record of clinical impact. As industry momentum increases, scalability hinges on the adoption of harmonized evaluation frameworks to streamline the process of bringing safe and effective digital therapeutics into clinical use globally.
To embark on the next era of industry growth, DTA released the “DTx Value Assessment & Integration Guide”- a first ever industry framework for the evaluation and implementation of digital therapeutics. (Available here.)
The Guide provides a tool for payors, policymakers, clinicians, and other healthcare decision makers (HCDMs) to use in assessing baseline information about DTx products, their value, and their impact in real-world settings. It was developed through a collaborative two-year process incorporating insights from DTA members, payors, clinicians, and other key stakeholders to ensure applicability across settings.
As a part of the launch, DTA hosted a public webinar to discuss the release of the “DTx Value Assessment & Integration Guide” with panelists:
- Megan Coder, PharmD, Chief Policy Officer at Digital Therapeutics Alliance
- Trina Histon, PhD, Senior Principal Consultant Prevention, Wellness & Digital Health at Kaiser Permanente
- Anand Iyer, Chief Strategy Officer at Welldoc
- Andrey Ostrovsky, MD, Former Chief Medical Officer of US Medicaid Program & Managing Partner of Social Innovation Ventures
- Nicole Owings-Fonner, MA, Director of Operations and Innovation at American Psychological Association
DTA Launches “DTx Value Assessment & Integration Guide” to Develop Industry-First Framework for Harmonization
Digital Therapeutics Alliance Launches “DTx Value Assessment & Integration Guide” to Develop Industry-First Framework for Harmonization
-
The Guide harmonizes expectations related to DTx product quality, security, clinical evaluation, and regulatory requirements
-
Developed through a collaborative two-year process via interviews and workshops with industry stakeholders globally
ARLINGTON, VA – May 17, 2022 – The Digital Therapeutics Alliance (DTA), a global non-profit trade association of industry leaders and stakeholders engaged in the evidence-driven advancement of digital therapeutics (DTx), announced today the industry’s first guidance on digital therapeutics best practices, the DTx Value Assessment & Integration Guide (“the Guide”).
This inaugural edition serves as a leading framework of evaluation and implementation of digital therapeutics (DTx), that use innovative, clinically-validated disease prevention, management, and direct treatment technologies to enhance, complement, and in some cases replace, current medical practices and treatments.
The Guide harmonizes expectations related to DTx product quality, security, clinical evaluation, and regulatory requirements and was developed through a collaborative two-year process incorporating insights from payors, clinicians, and manufacturers to ensure applicability across settings.
This Guide focuses on digital therapeutics—products that deliver clinical interventions directly to patients via software to treat, manage, or prevent a disease or disorder. DTx products may also integrate a variety of other digital capabilities into their product offerings, such as wearables and biometric sensors, diagnostic capabilities, the delivery of health information to patients, and clinical decision support features for clinicians.
Given the growth of the DTx industry, and as more healthcare decision makers (HCDMs) across the world review, assess, approve, and implement DTx products, it is important for clinicians, policy makers, and payors to have access to a reliable framework and industry guidance that enable consistent evaluation of DTx products across local, national, and regional settings.
DTA developed the DTx Value Assessment & Integration Guide to provide a common language and process for HCDMs and DTx manufacturers to jointly use throughout DTx product evaluation processes. By providing the building blocks of DTx product review and economic assessment pathways, this Guide serves as a tool for HCDMs and DTx manufacturers to use in assessing baseline information about DTx products, their value, and their impact in real-world settings.
“We founded the Digital Therapeutics Alliance to ensure that patients in every part of the world will be able to access and trust the safety, quality, and impact of DTx therapies,” said Megan Coder, DTA Chief Policy Officer. “In the last decade, the DTx industry has matured significantly, and this framework provides a timely blueprint to advance the digital therapeutics industry into the next era of innovation.”
The Guide incorporates feedback from payors, clinicians, and manufacturers from key international markets. It assists HCDMs, including payors, employers, governments, evaluators, health system administrators, clinical leaders, patients, and other individuals, in assessing, integrating, and scaling DTx products across clinical-use settings.
“This new guidance highlights the important role of private-public collaboration in a robust digital therapeutics economy,” said Trina Histon, PhD, Senior Principal Consultant Prevention, Wellness & Digital Health at Kaiser Permanente. “As clinicians, healthcare systems, employers, and payors continue integrating these products into patient care, digital therapeutics will increasingly augment the delivery and consumption of healthcare globally. The DTA guidance will serve as a reference for DTx companies to adopt a consistent set of recommended industry standards to bring more innovation to patients.”
“For over 5 years, Digital Therapeutics Alliance has led national-level policymaking and its direct impact on the life sciences industry,” said Owen McCarthy, Chair Board of Directors, DTA and President & Co-Founder, MedRhythms. “This inaugural guidance offers a much-needed framework for the public, policymakers, and others to understand the many elements that are necessary to continue to drive the digital therapeutics industry forward.”
This Guide serves as a critical step in product access and contracting discussions. It addresses a wide spectrum of HCDM considerations across various settings—ranging from individual health systems and employers to single-payor government systems, and multi-payor public/private settings—and will continue to be updated to ensure ongoing relevance in this quickly evolving ecosystem. Learn more and download the Guide.
About Digital Therapeutics Alliance
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
Media Contact:
Hannah Fairman
hfairman@dtxalliance.org
DTA Convened Industry Leaders for Event on Capitol Hill to Advocate for Policy and Legislation to Provide Better Access and Coverage for Digital Therapeutics
DTA Convened Industry Leaders for Event on Capitol Hill to Advocate for Policy and Legislation to Provide Better Access and Coverage for Digital Therapeutics
Participants engaged with policy makers to discuss avenues to expand access to digital therapeutics to improve clinical outcomes and reduce disparities for mental health care
April 7, 2022 – Arlington, VA – This week, the Digital Therapeutics Alliance (DTA) convened industry leaders from the U.S. Policy Task Group, including several DTA Board members for an education and advocacy conference in Washington, DC to meet with lawmakers, federal experts, and key congressional committee staff to discuss policy and legislative opportunities to provide better access and coverage for digital therapeutics (DTx) and discuss next steps for the “Access to Prescription Digital Therapeutics Act”.

As the leading international organization on digital therapeutic thought leadership and education, DTA is a strong advocate for expanded access to DTx products to improve clinical and health economic outcomes. As a part of this work, DTA is engaging directly with federal officials to ensure that patients, caregivers, and clinicians are able to access and benefit from DTx therapies.
The two-day event brought leaders in the digital therapeutics industry together from across the U.S. to educate policy makers about how digital therapeutics are revolutionizing healthcare, and to learn about how the industry can best move forward with CMS reimbursement.
During the event, guest speakers engaged participants in a discussion on the current political environment and other relevant topics, including the legislative and regulatory process, digital health and healthcare innovation policy, and opportunities to reduce disparities with more equitable access to care.
DTA and representatives from member organizations engaged in meetings with lawmakers on Capitol Hill to urge passage of legislation to enable expanded access to digital therapeutics through the “Access to Prescription Digital Therapeutics Act” along with other related issues. This bipartisan legislation would establish benefit categories for a subset of digital therapeutics, and if passed, would provide a much needed access pathway for the over 160 million people covered by Medicare, Medicaid, and other publicly funded programs.
This legislation comes at a critical moment for patients and the U.S. healthcare system, when the need for better access to care for chronic conditions – and especially mental health care – is growing across all ages. In addition, many are not able to access needed care, either due to geographic limitations, cultural and language boundaries, growing disparities in access and coverage, health condition severity, or demand far exceeding the supply of qualified clinicians.
The lack of regulatory action to clarify a CMS benefit category and the patchwork of commercial coverage options have meant too few– and especially those with public coverage– have been able to benefit from DTx therapies. This legislation would close that gap and would build on other recent positive developments, including the American Medical Association CPT Editorial Panel’s decision to establish a new Current Procedural Terminology (CPT) code for remote monitoring of cognitive behavioral therapy and CMS’ action to establish the first Level II Healthcare Common Procedure Coding System (HCPCS) code for prescription digital behavioral therapy.
“Digital Therapeutics have a lot of momentum right now, especially with the Access to a Prescription Digital Therapeutics Act,” says Andy Molnar, DTA Chief Executive Officer. “The need for a benefit category and proper coding will allow for patients and providers to own their care.”

Pictured from left to right: Susan Stoner Zook – Chief Executive Officer and Founder of Mason Street Consulting, Beth Keyt – VP, Government Affairs at Pear Therapeutics, Everett Crosland – Chief Commercial Officer at Cognito Therapeutics, Andy Molnar – Chief Executive Officer at Digital Therapeutics Alliance, Sara Elalamy – U.S Government Affairs Director at Digital Therapeutics Alliance, Benjamin Alouf – Chief Medical Officer at Limbix, Anand Iyer – Chief Strategy Officer at WellDoc, Bob Cuyler – Chief Clinical Officer at Freespira, Jacqueline Studer – Chief Legal Officer at Akili interactive, Owen McCarthy – President and Co- Founder at MedRhythms, Will Robinson – Head of Policy and Strategy at Big Health
As we expand efforts to establish clearer pathways for DTx products to improve patient outcomes, DTA will be engaging members in additional advocacy efforts. Connect with us if you would like to be involved in these efforts.
Special thanks go to DTA Resource Partner, Susan Stoner Zook, Mason Street Consulting was instrumental in planning and executing this conference.
DTA engaging in policy discussions to advocate for greater recognition of digital therapeutics in US policies
DTA engaging in policy discussions to advocate for greater recognition of digital therapeutics in national policies in the United States
With recent comments on proposed rules and regulations, DTA is ensuring members and the patients they serve are considered in policy decisions
March 24, 2022 – Arlington, VA – The Digital Therapeutics Alliance (DTA) is leading policy and advocacy efforts to expand adoption, coverage, and access to clinically validated digital therapeutics (DTx). During a critical time, this innovative category of medicine is addressing unmet needs and major gaps in healthcare. The pandemic and subsequent challenges have resulted in many patients unable to access much needed care – either due to geographic limitations, cultural and language barriers, well-documented disparities, health condition severity, or demand far exceeding the supply of qualified clinicians.
DTA is working with policymakers to support the wider integration of DTx therapies across the healthcare ecosystem to ensure these evidence-based treatments get into the hands of those who need them most.
DTA’s current advocacy efforts in the United States are focused on:
- Formal recognition of DTx products: Officially define and recognize DTx products in legislation and regulation so Medicare and Medicaid patients can access these critical therapies.
- Codifying DTx product coverage: Require CMS to assure that Medicare and Medicaid cover technologies that meet the definition of a digital therapeutic.
- Expanding clinician coding and payment: Direct CMS to ensure that adequate payment mechanisms exist to pay primary care providers and clinicians engaged in the authorization and clinical use of DTx products.
By engaging directly with federal agency officials and submitting comments on proposed rules and regulations, DTA is working to ensure members and the patients they serve are considered in policy decisions.
As a part of these efforts, this week DTA submitted comments related to two Food and Drug Administration draft guidances:
- DTA response to ‘Transition Plan for Medical Devices That Fall Within Enforcement Policies Issued During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency’ (FDA-2021-D-1118), published in December 23, 2021.
- DTA response to ‘Digital Health Technologies for Remote Data Acquisition in Clinical Investigations’ (FDA-2021-D-1128), published December 21, 2021.
Additional recent responses from DTA include:
February 28, 2022 – DTA responds to the White House Office of Science and Technology Policy (OSTP) Request for Information (RFI) on Strengthening Community Health Through Technology (87 FR 492), published January 5, 2022.
February 28, 2022 – DTA responds to the Food and Drug Administration’s ‘Considerations for the Use of Real-World Data and Real-World Evidence To Support Regulatory Decision-Making for Drug and Biological Products,’ (FDA-2021-D-1214) published December 9, 2021.
February 28, 2022 – DTA responds to the Food and Drug Administration’s ‘Real-World Data: Assessing Registries to Support Regulatory Decision-Making for Drug and Biological Products,’ (FDA-2021-D-1146) published November 30, 2021.
January 15, 2022 – DTA responds providing an overview of efforts to ensure product safety and reliability for the White House Office of Science and Technology Policy (OSTP) Request for Information on public and private uses of biometric technologies (86 FR 56300), published October 8, 2021.
November 29 , 2021- DTA responds to the Agency for Healthcare Research and Quality’s (AHRQ) draft technical brief “Evaluation of Mental Health Mobile Applications.” (86 FR 28601), published October 20, 2021.
November 26, 2021 – DTA responds to Food and Drug Administration’s ‘Notice to Public of Website Location of CDRH Fiscal Year 2022 Proposed Guidance Development,’ (FDA-2012-N-1021), published October 26, 2021.
DTA keeps members informed about all relevant federal actions in the U.S. as well as provides opportunities for members to directly engage in advocacy efforts through its US Policy Task Group, member newsletter, and Slack community. Connect with us if you would like to be involved in these efforts.
DTA Announces 2022 Task Groups and Co-Chairs
DTA Announces 2022 Task Groups and Co-Chairs
Engagement from industry leaders will provide critical support on executing DTA’s 2022 priorities
Tuesday, March 22, 2022 – Arlington, VA – This year, The Digital Therapeutics Alliance (DTA) is leading initiatives focused on three priorities — at the international, national, and regional levels — to drive the industry forward.

DTA members and Resource Partners are spearheading our efforts to transform global healthcare through seven newly launched Task Groups.
Asia Pacific Policy Task Group
Focus: This group focuses on key aspects of the Asia-Pacific policy landscape, including regulatory frameworks and national funding pathways.
DTA Member Co-Chairs:
- Roger Darlington, Business Development Manager at Mundipharma
- Sirius Baiyan Ge, Director of Digital Therapeutics Innovation at Weimai
- Danny Kim, Chief of Staff at WELT
Clinical Evidence Task Group
Focus: This group is developing DTx-specific guidelines and core criteria for healthcare decision maker clinical evidence evaluation processes.
DTA Member Co-Chairs:
- Taehwa Han, Research Professor at Yonsei University Health System
- Vandana Menon, VP of Clinical Research and Evidence Generation at Akili Interactive
- Dario Motti, Global Principal Scientist Digital Health Technologies at Roche
- Jeanette Waxmonsky, Director of Research at Big Health
Resource Partners:
- Curebase
- Sidley Austin, LLP
DTx Value Assessment & Integration Guide Task Group
Focus: This group is guiding the launch and update phases of DTA’s ‘DTx Value Assessment & Integration Guide,’ which establishes a framework to consistently evaluate and benchmark DTx products.
DTA Member Co-Chairs:
- Taehwa Han, Research Professor at Yonsei University Health System
- Jeanette Waxmonsky, Director of Research at Big Health
Resource Partner:
Europe Policy Task Group
Focus: This group focuses on key aspects of the European policy landscape, including Health Technology Assessment (HTA) frameworks, national funding, and patient access pathways.
DTA Member Co-Chairs:
- Helene Moore, General Manager at Ethypharm Digital Therapy
- Mikaela Odlander, Director and Head of Digital Therapeutics at Orexo
Public Relations & Communications Task Group
Focus: This group is developing resources to promote broader DTx awareness and understanding, in addition to addressing industry-level challenges.
DTA Member Co-Chairs:
- Danny Kim, Chief of Staff at WELT
- Anni Mansikkaoja, Marketing & Communications Manager at Kaiku Health
- Jake Mazanke, Head of Communications at Big Health
- Lauren Steidl, Corporate Communications Lead at MedRhythms, Inc.
US Commercialization & Reimbursement Task Group
Focus: This group is working to establish payment pathways in the United States by addressing coding and coverage issues, in addition to developing product commercialization resources.
DTA Member Co-Chairs:
- Aaron Gani, Founder & CEO at BehaVR
- Joseph Perekupka, Representing MedRhythms
Resource Partners:
- ClearView Healthcare Partners
US Policy Task Group
Focus: This group is focused on DTA’s ongoing work related to U.S. legislation and regulatory efforts with federal agencies.
DTA Member Co-Chairs:
- Michael Pace, Representing Woebot Health
- Will Robinson, Head of Policy and Strategy at Big Health
Resource Partners:
As DTA continues to lead the digital therapeutic industry, these collective efforts will be instrumental in providing patients and caregivers, clinicians, policymakers, and healthcare decision makers with reliable resources to meaningfully integrate DTx products into practice to improve clinical and health economic outcomes globally.
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
 September 26, 2022 – Arlington, VA– This past week, the Digital Therapeutics Alliance (DTA) held a Capitol Hill Advocacy Event in Washington, DC for member companies and allied partners to discuss policy and legislative opportunities to provide better access and coverage for digital therapeutics (DTx) and gain support for H.R. 7051/ S. 3791 the “Access to Prescription Digital Therapeutics Act of 2022”.
September 26, 2022 – Arlington, VA– This past week, the Digital Therapeutics Alliance (DTA) held a Capitol Hill Advocacy Event in Washington, DC for member companies and allied partners to discuss policy and legislative opportunities to provide better access and coverage for digital therapeutics (DTx) and gain support for H.R. 7051/ S. 3791 the “Access to Prescription Digital Therapeutics Act of 2022”.