
Arlington, VA and San Francisco, CA – Tuesday, December 6, 2022 – The Digital Therapeutics Alliance (DTA), a global non-profit trade association with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare, in collaboration with DTA Resource Partner, Curebase, a company committed to democratizing access to clinical studies, today released a publication to provide a fit-for-purpose evidence standard for DTx product regulatory, reimbursement, and clinical acceptance.
The publication, “Setting the Stage for a Fit-For-Purpose DTx Evidentiary Standard”, outlines foundational principles specific to the DTx category of medicine and baseline expectations for healthcare decision makers (HCDMs) related to the types, quality, and timing of clinical trials necessary to evaluate and implement DTx therapies in real-world settings.
Given the profound growth of the DTx industry over the last decade, and as more HCDMs across the world embark on evaluating DTx products, it is important for clinicians, policymakers, and payors to have access to a harmonized, consistent set of study expectations to sufficiently demonstrate product safety, efficacy, and impact. Thus far, DTx products have been subject to the same clinical evidence standards that are used to assess the safety and effectiveness of other traditional medical devices and pharmaceuticals. However, given the unique nature of DTx products, it is increasingly clear that a fit-for-purpose evidence evaluation standard is required to account for the faster and iterative nature of DTx product life cycles, levels of potential risk, and their place in clinical therapy.
“For broader patient access to DTx therapies across the global marketplace, evaluation frameworks need to be clear, consistent, and specific to the unique characteristics of DTx products,” said Megan Coder, Chief Policy Officer of the Digital Therapeutics Alliance. “A fit-for-purpose DTx product evidence evaluation framework – reflective of how DTx products are designed and used in real-world settings – strengthens DTx evidentiary robustness and clarity, thereby preventing unnecessary delays in patient access to high-quality clinically-validated digital therapeutics.”
“Patients everywhere deserve access to high-quality care; having a fit-for-purpose evidence standard for DTx brings the industry one step closer to this,” says Whitney Stewart, Director of Clinical Project Management at Curebase. “By aligning the industry and providing the foundation for DTx studies, this paper paves the way for more thorough DTx studies, for a clearer path forward for DTx companies, and should result in overall better patient care.”
This publication provides evidence-based expectations for how DTx products should be validated by payors globally, building on principles from pharmaceutical and general medical device standards, while acknowledging digital therapeutics’ unique mechanisms of action, iterative development lifecycles, and clinical outcomes. To enable movement toward a fit-for-purpose DTx evidentiary standard, DTA will continue to engage with the broader healthcare ecosystem to develop harmonized and DTx-specific clinical evidence frameworks. For more information, and to download the paper, visit: https://dtxalliance.org/wp-content/uploads/2022/12/DTA-Clinical-Evidence-Paper_12.22.pdf.
###
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors in order to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.
About Curebase
At Curebase, our mission is to bring quality medical innovations to patients faster and improve human well-being through more efficient clinical studies. We are proving that clinical research can be radically accelerated if we empower physicians everywhere to enroll patients in the communities where they live. By applying cutting-edge clinical software and remote study management techniques to the problem, we are reinventing clinical trials and research from the ground up. For more information, please visit www.curebase.com.
Authors: Steven Driver MD, Di Jiang, Alona May, Lani Hessen, Andy Molnar

Remote Physiologic Monitoring (RPM) and Remote Therapeutic Monitoring (RTM) codes are playing an increasingly important role in the adoption and uptake of digital health technologies, particularly digital therapeutics (DTx). DTx products – evidence-based therapeutic interventions driven by high quality software to prevent, manage, or treat a medical disorder or disease – frequently generate physiologic and therapeutic response data. Clinicians use RPM and RTM Current Procedural Terminology (CPT®) codes to account for time spent reviewing and assessing DTx-generated data, thus making these payment codes essential to clinician adoption of these technologies.1
RPM refers to the use of digital technologies to capture and analyze patients’ physiological data, such as blood pressure, glucose levels, and lung function. By contrast, RTM refers to codes that capture and analyze outcomes such as therapy response and patient adherence related to treatment (musculoskeletal, cognitive behavioral therapy and respiratory). In the case of RTM, clinicians are able to bill for their review of non-physiologic medical device data that is generated by digital technologies.
RPM codes, established by CMS in 2018, establish coverage and payment for remote monitoring of patient data. In the same year, the American Medical Association developed CPT codes for remote physiologic monitoring (RPM) equipment and companion treatment management services (TMS). In 2019, CMS covered and paid for RPM and RPM-TMS codes. Over the ensuing years, CMS has continued to refine their coverage and payment policies related to these codes, along with additional non-face-to-face care management services.
As the use of remote care continues to increase, healthcare leaders need to understand coding to account for the supply of RPM and RTM equipment as well as professional time working with patients using remote methods.
Varying forms of remote monitoring and their uses in clinical care have existed for decades. The Covid-19 pandemic only helped to underscore its importance. As service waivers from the pandemic are now transitioning, decision makers are determining how to continue their ongoing use. However, RPM and RTM along with the recent creation of a Healthcare Common Procedure Coding System (HCPCS) Level II supply code are not temporary and signal permanent direct reimbursement mechanisms.
This publication discusses the impact that the Covid-19 pandemic had on clinicians’ utilization of RPM and RTM codes, and sheds light on the increasing trajectory of clinician use of remotely delivered care solutions.
The analysis was primarily conducted using Komodo’s patient cohort creation platform, Prism, and supplemented with additional analysis from Komodo’s Healthcare Map. For each set of codes (RPM and RTM), queries were run to identify the healthcare professionals (HCPs) using the codes and further segment them by specialty and location. In consideration of the length of time each code set has been available to providers, uptake over the COVID pandemic was assessed for RPM and initial post-launch uptake was assessed for RTM. Each analysis (RPM and RTM) was executed looking at the entire code set – not specific to any individual code within the set.
For the RPM landscape analysis, the following codes were queried from Komodo’s Healthcare Map using the Prism software.
| Code | Code Type | Description |
|---|---|---|
| 99453 | CPT | Remote monitoring of physiologic parameter(s) (eg, weight, blood pressure, pulse oximetry, respiratory flow rate), initial; set-up and patient education on use of equipment |
| 99454 | CPT | Remote monitoring of physiologic parameter(s) (eg, weight, blood pressure, pulse oximetry, respiratory flow rate), initial; device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days |
| 99457 | CPT | Remote physiologic monitoring treatment management services, clinical staff/physician/other qualified health care professional time in a calendar month requiring interactive communication with the patient/caregiver during the month; first 20 minutes |
| 99458 | CPT | Remote physiologic monitoring treatment management services, clinical staff/physician/other qualified health care professional time in a calendar month requiring interactive communication with the patient/caregiver during the month; first 20 minutes, each additional 20 minutes |
| 99091 | CPT | Collection and interpretation of physiologic data (eg, ECG, blood pressure, glucose monitoring) digitally stored and/or transmitted by the patient and/or caregiver to the physician or other qualified health care professional, qualified by education, training, licensure/regulation (when applicable) requiring a minimum of 30 minutes of time, each 30 days |
| 95250 | CPT | Ambulatory continuous glucose monitoring of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours; physician or other qualified health care professional (office) provided equipment, sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording |
| 99473 | CPT | Self-measured blood pressure using a device validated for clinical accuracy; patient education/training and device calibration |
| 99474 | CPT | Self-measured blood pressure using a device validated for clinical accuracy; separate self-measurements of two readings one minute apart, twice daily over a 30-day period (minimum of 12 readings), collection of data reported by the patient and/or caregiver to the physician or other qualified health care professional, with report of average systolic and diastolic pressures and subsequent communication of a treatment plan to the patient |
Covid-19 sped up the already positive uptake of RPM codes by clinicians. The majority (64%) of providers started using RPM codes for the first time during Covid. Towards the end of 2022, nearly 10,000 clinicians, referred to in Figure 1 as healthcare providers (HCPs), were utilizing the RPM codes that were examined in the analysis.
Figure 1.
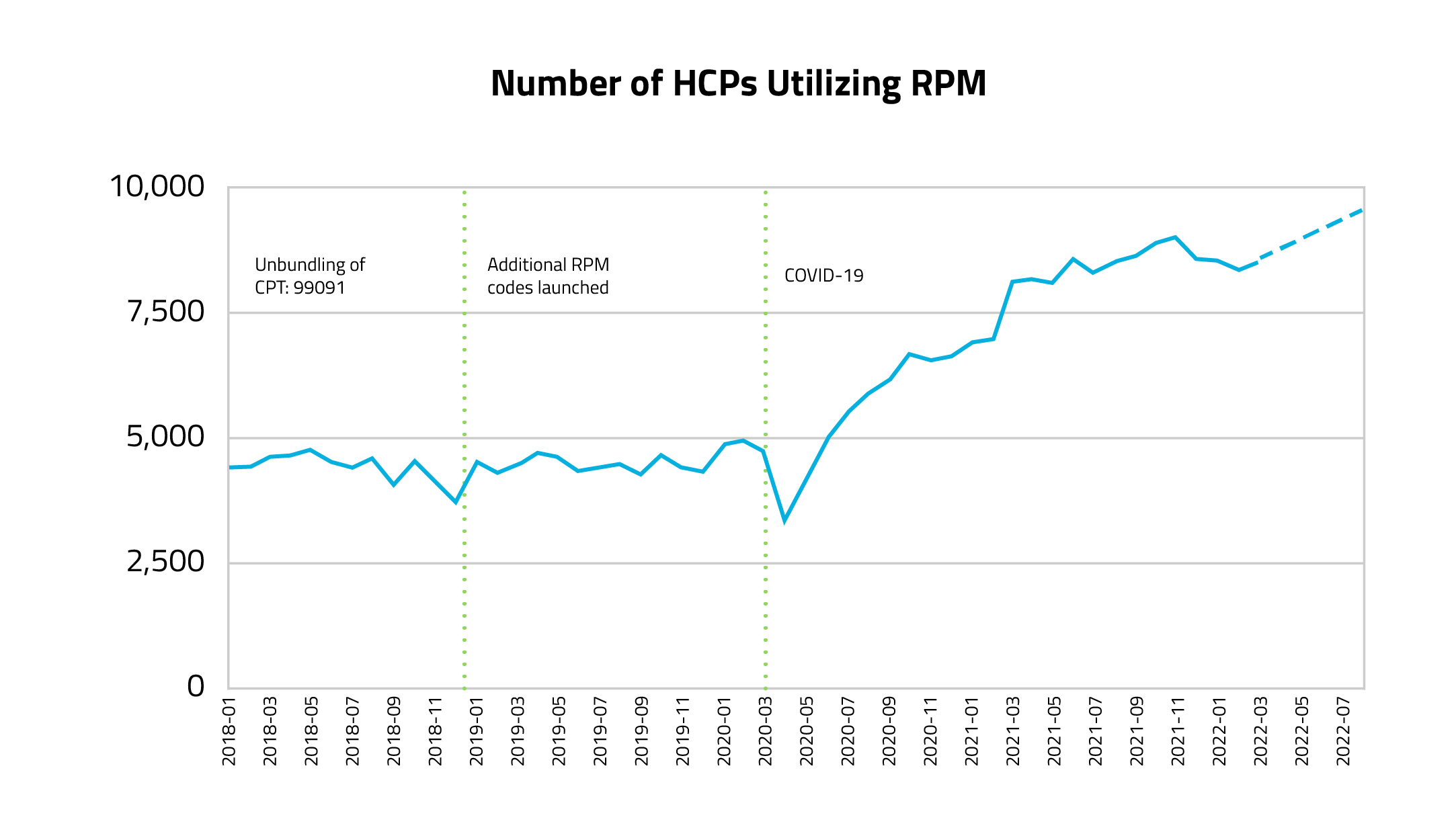
Figure 1 – Created using Komodo’s Healthcare Map, this graph demonstrates how many HCPs billed using RPM codes each month during the time period of Jan 2018 – Mar 2022 (projected to Jul 2022). The query was completed by pulling the number of unique HCPs who used any of the RPM codes each month from de-duplicated medical claims. HCPs may repeat month by month (if using the codes consistently). Steady growth was evident after the start of the COVID pandemic and to account for claim lag while reviewing initial numbers for the last 4 months of activity, a positive projected slope was deduced.
Over the last four years, 50,000 unique HCPs located at over 19,000 healthcare organizations have billed for an RPM code. Of these HCPs, 64% began using the codes during the pandemic (starting March 2020). Prior to Covid, the use of RPM codes remained fairly stable, but following the start of the pandemic, there was a significant surge in RPM usage, effectively doubling the number of HCPs using these codes per month.
As demonstrated in Figure 2, the most frequent types of clinicians using RPM codes are community-based primary care clinicians, such as family practitioners, internal medicine doctors, and nurse practitioners. Additionally, as seen in Figure 3, cardiologists, nephrologists, gastroenterologists, and psychiatrists made up the majority of the new adopters of RPM codes during the Covid era (represented by yellow arrows).
Figure 2.
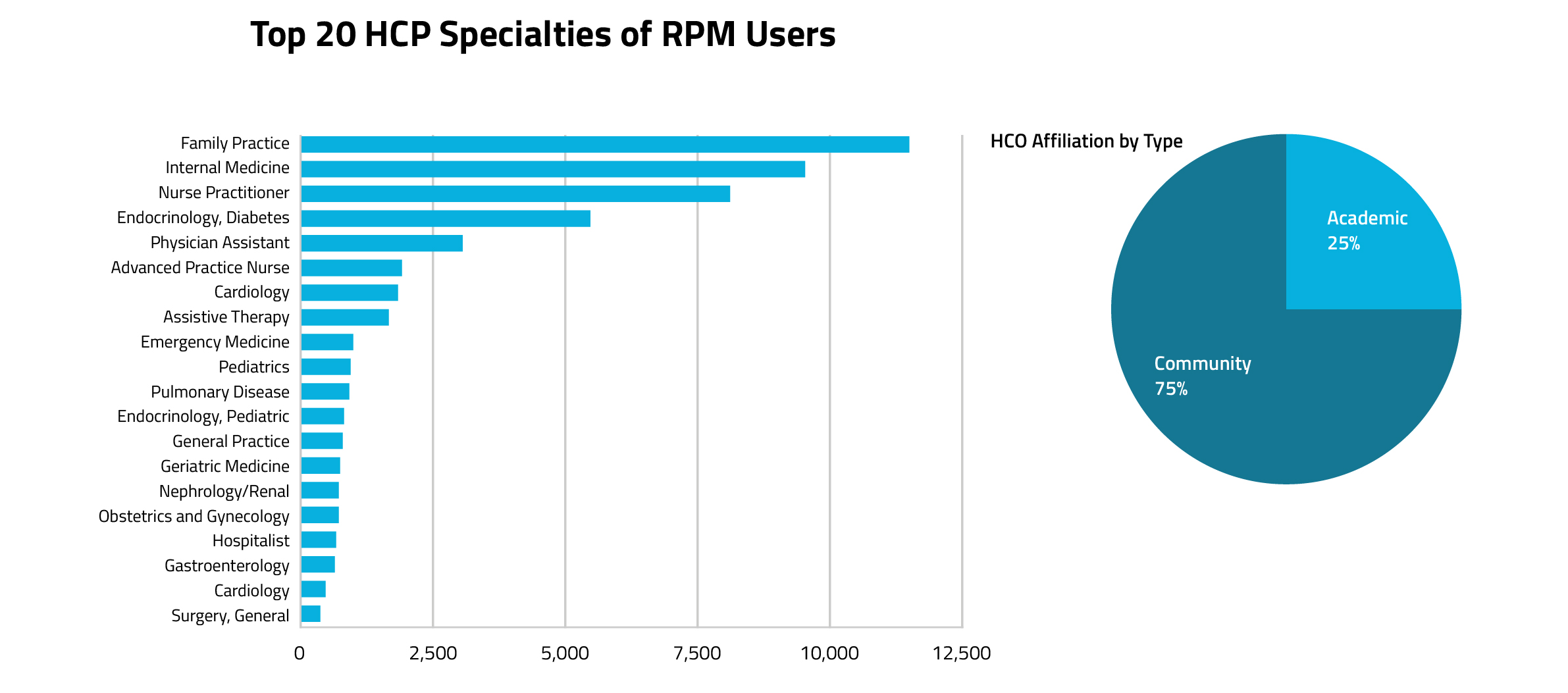
Figure 3.
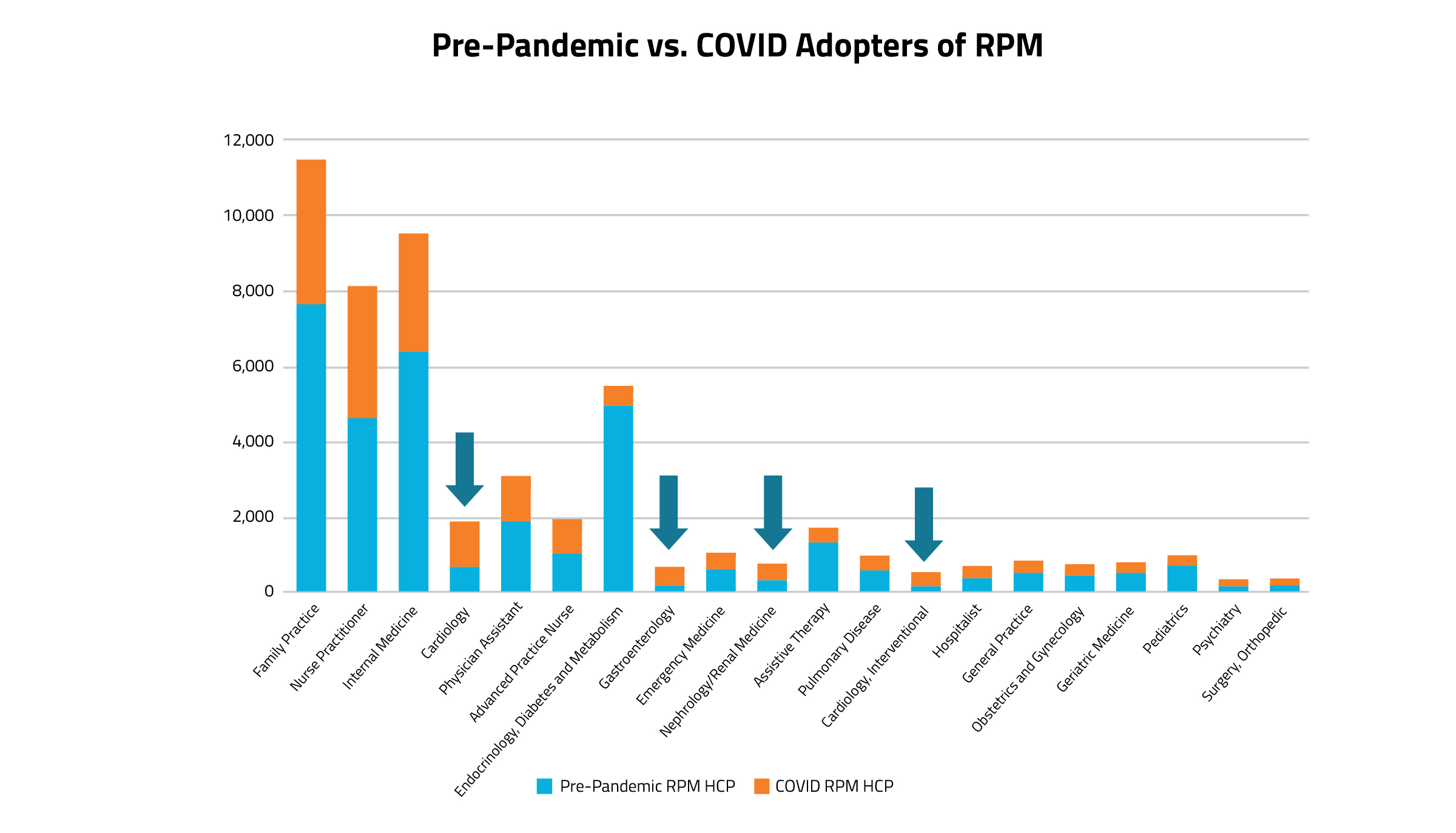
When analyzing the data by clinician specialty, patterns begin to emerge. Of the top twenty specialties who most commonly use RPM codes, many are primary care clinicians. Of all the clinician specialty groups who use RPM codes in practice, only a select few have a larger proportion of Covid adopters vs pre-Covid users: cardiologists (including interventional cardiology), nephrologists, gastroenterologists, and psychiatry. Even though primary care specialists tower over the others in terms of the sheer volume of RPM code usage, the majority of primary care providers who used RPM codes during Covid billed at least once for an RPM code prior to the pandemic.
As more primary care specialists (particularly internal medicine) became consistent users of RPM codes during the pandemic era, there was a significant uptake in unique HCP counts.
For the RTM landscape analysis, the following codes were queried from Komodo’s Healthcare Map using the Prism software.
| Code | Code Type | Description |
|---|---|---|
| 98975 | CPT | Remote therapeutic monitoring (eg, respiratory system status, musculoskeletal system status, therapy adherence, therapy response); initial set-up and patient education on use of equipment |
| 98976 | CPT | Remote therapeutic monitoring (eg, respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (eg, daily) recording(s) and/or programmed alert(s) transmission to monitor respiratory system, each 30 days |
| 98977 | CPT | Remote therapeutic monitoring (eg, respiratory system status, musculoskeletal system status, therapy adherence, therapy response); device(s) supply with scheduled (eg, daily) recording(s) and/or programmed alert(s) transmission to monitor musculoskeletal system, each 30 days |
| 98980 | CPT | Remote therapeutic monitoring treatment management services, physician or other qualified health care professional time in a calendar month requiring at least one interactive communication with the patient or caregiver during the calendar month; first 20 minutes |
| 98981 | CPT | Remote therapeutic monitoring treatment management services, physician or other qualified health care professional time in a calendar month requiring at least one interactive communication with the patient or caregiver during the calendar month; each additional 20 minutes (List separately in addition to code for primary procedure) |
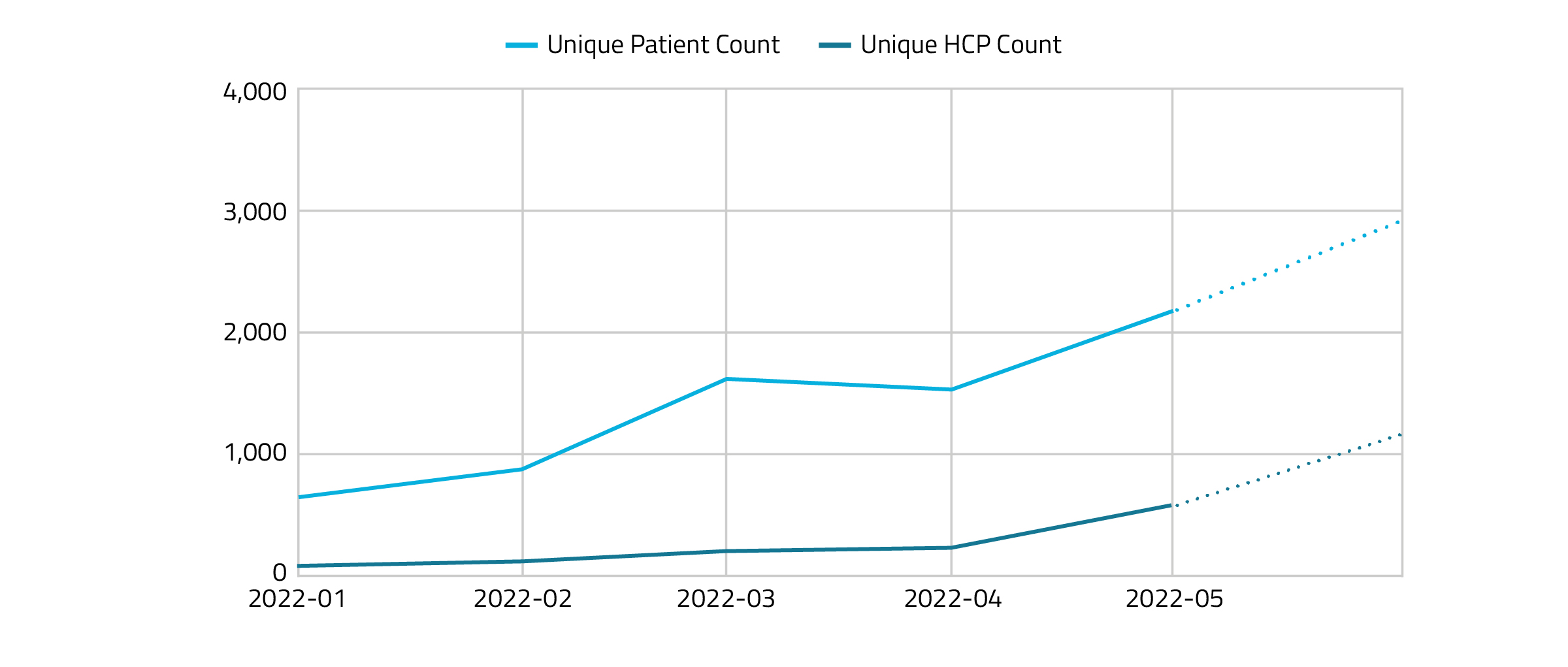
Figure 4 shows the RTM HCP and patient use per month starting in January 2022. The use of RTM codes has continued to steadily increase over the first 5 months.
Figure 4.
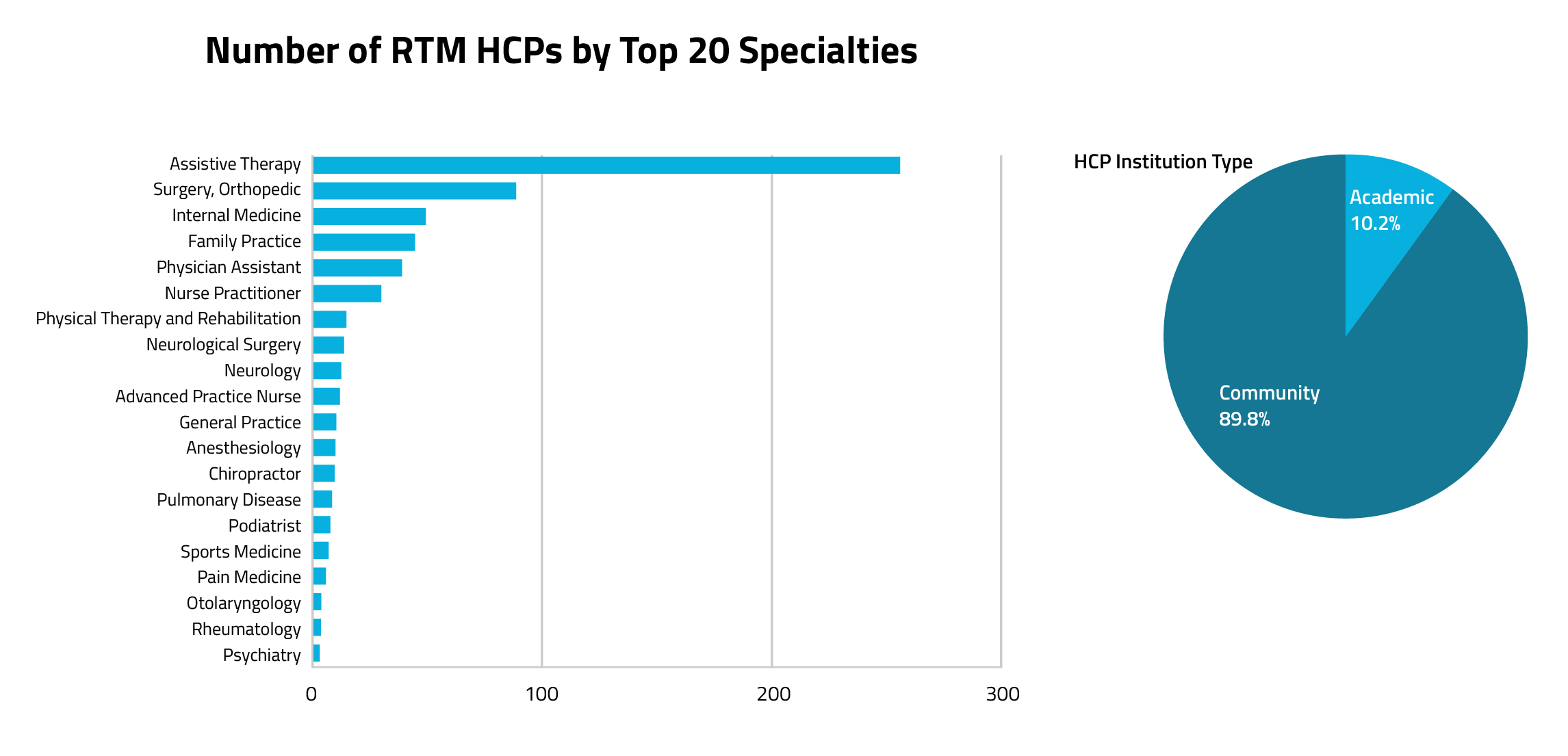
Overall, physicians make up the largest group of RTM code users, according to Figure 5. Community-Based Assistive Therapy Specialists, such as physical and occupational therapists, have been the most common clinician group to adopt RTM codes.
Figure 5.
Although long-term data is limited given the recent release of these codes (i.e., RTM and RTM-TMS coverage became effective January 1, 2022), a conservative uptake is being noted. It is important to continue tracking usage as time goes on.
While primary care clinicians are still frequent RTM code users, assistive therapy specialists are by far the most common RTM code users. In comparing HCP institution types, a majority of RPM users were found in community-based organizations versus academic organizations, This is similar to the institution split for RPM codes. Also like RPM code utilization trends, primary care clinicians serve as the top specialists for this code cohort.
Translating new evidence into widespread clinical implementation typically requires approximately 17 years1. However, the Covid-19 pandemic rapidly accelerated the adoption of digital health technologies, in particular those that enabled remote physiologic monitoring and remote therapeutic monitoring. While certain digital health tools such as telehealth visits initially spiked early in the pandemic they subsequently declined.2 The utilization of RPM and RTM codes observed a more durable and steady increase in adoption over time. This suggests that clinicians and patients perceive the high worth of RPM/RTM codes, in addition to CMS rightly compensating for the associated value.
Similarly, Congress is considering creation of Medicare and Medicaid benefit categories for another class of digital health tools via the Access to Prescription Digital Therapeutics Act3. Digital therapeutics are currently regulated by the FDA as medical devices, but not reimbursed by CMS in a defined benefit category. This presents a challenge for health systems which might otherwise be eager to get such evidence-based tools into the hands of their patients. While consumers and clinicians wait for an act of Congress to promote widespread adoption of these tools, RPM and RTM codes could help.
Many digital therapeutic interventions generate significant physiologic and therapeutic data including weight, blood pressure, and data related to treatment adherence. If captured, such data could be used by clinicians for RPM and RTM billing as a means of offsetting what can otherwise be cost prohibitive implementation, especially among fee-for-service populations. Digital therapeutic companies wishing to transition from the employee space dominated by insurers and large self-insured employers would do well to understand coding, coverage, and payment available to prescribers who could otherwise be ready partners in a broadening digital health ecosystem.
Education around the use of these codes enables clinicians to feel more confident in prescribing a DTx because the codes support that prescription. We observe a cohort of early adopters in the data, but clearly as uptake is generally slow for new codes, we are also dealing with a still nascent industry. Major updates are expected to the RPM-TMS codes as CMS has proposed through 2023 rulemaking to dramatically affect RTM-TMS codes. Should these proposals be finalized, it will affect the current makeup and utilization of these codes.
All new CPT codes, including RPM and RTM codes, take time to see broad adoption, or any adoption at all. Their creation seeks to address gaps in services and reimbursement for clinicians’ time that specifically allows them to provide better care for their patients. These gaps exist with government and commercial payors.
Considering technological advances in healthcare, adoption can be difficult for a variety of reasons including technological resistance, education, etc., but also from a coding perspective.
Looking at the impact of Covid to the use of technology in healthcare, it is very encouraging to finally see that technology can be adopted at a faster rate. Clinicians are seemingly willing to adopt new technologies.
Looking at recent utilization, we’re starting to see a new industry take hold.
Keywords: Digital therapeutics, Remote Physiologic Monitoring (RPM), Remote Therapeutic Monitoring (RTM), Medicare, Medicaid, Current Procedural Terminology (CPT®) code, Healthcare Common Procedure Coding System (HCPCS) code, Centers for Medicare & Medicaid Services (CMS)
1CPT© Copyright 2022 American Medical Association. All rights reserved. AMA and CPT are registered trademarks of the American Medical Association.
“Building A Regulatory And Payment Framework Flexible Enough To Withstand Technological Progress” by David Flannery and Robert Jarrin; 10.1377/hlthaff.2018.05151
Tuesday, November 8, 2022 – Arlington, VA – Today, the Digital Therapeutics Alliance (DTA), is pleased to announce the results of the 2023 DTA Board of Directors election. With two seats up for election this cycle, DTA members selected Danny Kim, Head of WELT USA, as a new board member and re-elected Owen McCarthy, Co-Founder and President of MedRhythms, Inc. and current Chair to serve on the Board.
The 2023 DTA Board of Directors:
The Board of Directors supports DTA’s work to transform global healthcare by advancing digital therapeutics (DTx) and provides critical guidance related to the strategy and direction of the organization.
“We are looking forward to working with this Board to capitalize on the organizational initiatives already underway and tackle additional innovative efforts to advance digital therapeutics,” said Andy Molnar, DTA’s Chief Executive Officer. “These industry leaders will draw on their experience building successful companies to provide crucial support guiding DTA’s work to expand patient access to DTx products to improve clinical and health economic outcomes.”
DTA’s Board has set the strategic vision to transform global healthcare by advancing digital therapeutics and their oversight and guidance will be instrumental in ensuring successful execution:
Debra Reisenthel, Vice-Chair of the DTA Board of Directors, shares, “Strong leadership from industry trailblazers has been essential to support the rapid evolution of the DTx industry as well as the considerable growth of the Alliance, which now represents over 100 companies across 20 countries. I am excited to work with my fellow directors to pursue our strategic focus in 2023 on advancing adoption and integration of digital therapeutics on a global scale.”
###
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors in order to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.