
April 7, 2022 – Arlington, VA – This week, the Digital Therapeutics Alliance (DTA) convened industry leaders from the U.S. Policy Task Group, including several DTA Board members for an education and advocacy conference in Washington, DC to meet with lawmakers, federal experts, and key congressional committee staff to discuss policy and legislative opportunities to provide better access and coverage for digital therapeutics (DTx) and discuss next steps for the “Access to Prescription Digital Therapeutics Act”.

As the leading international organization on digital therapeutic thought leadership and education, DTA is a strong advocate for expanded access to DTx products to improve clinical and health economic outcomes. As a part of this work, DTA is engaging directly with federal officials to ensure that patients, caregivers, and clinicians are able to access and benefit from DTx therapies.
The two-day event brought leaders in the digital therapeutics industry together from across the U.S. to educate policy makers about how digital therapeutics are revolutionizing healthcare, and to learn about how the industry can best move forward with CMS reimbursement.
During the event, guest speakers engaged participants in a discussion on the current political environment and other relevant topics, including the legislative and regulatory process, digital health and healthcare innovation policy, and opportunities to reduce disparities with more equitable access to care.
DTA and representatives from member organizations engaged in meetings with lawmakers on Capitol Hill to urge passage of legislation to enable expanded access to digital therapeutics through the “Access to Prescription Digital Therapeutics Act” along with other related issues. This bipartisan legislation would establish benefit categories for a subset of digital therapeutics, and if passed, would provide a much needed access pathway for the over 160 million people covered by Medicare, Medicaid, and other publicly funded programs.
This legislation comes at a critical moment for patients and the U.S. healthcare system, when the need for better access to care for chronic conditions – and especially mental health care – is growing across all ages. In addition, many are not able to access needed care, either due to geographic limitations, cultural and language boundaries, growing disparities in access and coverage, health condition severity, or demand far exceeding the supply of qualified clinicians.
The lack of regulatory action to clarify a CMS benefit category and the patchwork of commercial coverage options have meant too few– and especially those with public coverage– have been able to benefit from DTx therapies. This legislation would close that gap and would build on other recent positive developments, including the American Medical Association CPT Editorial Panel’s decision to establish a new Current Procedural Terminology (CPT) code for remote monitoring of cognitive behavioral therapy and CMS’ action to establish the first Level II Healthcare Common Procedure Coding System (HCPCS) code for prescription digital behavioral therapy.
“Digital Therapeutics have a lot of momentum right now, especially with the Access to a Prescription Digital Therapeutics Act,” says Andy Molnar, DTA Chief Executive Officer. “The need for a benefit category and proper coding will allow for patients and providers to own their care.”

Pictured from left to right: Susan Stoner Zook – Chief Executive Officer and Founder of Mason Street Consulting, Beth Keyt – VP, Government Affairs at Pear Therapeutics, Everett Crosland – Chief Commercial Officer at Cognito Therapeutics, Andy Molnar – Chief Executive Officer at Digital Therapeutics Alliance, Sara Elalamy – U.S Government Affairs Director at Digital Therapeutics Alliance, Benjamin Alouf – Chief Medical Officer at Limbix, Anand Iyer – Chief Strategy Officer at WellDoc, Bob Cuyler – Chief Clinical Officer at Freespira, Jacqueline Studer – Chief Legal Officer at Akili interactive, Owen McCarthy – President and Co- Founder at MedRhythms, Will Robinson – Head of Policy and Strategy at Big Health
As we expand efforts to establish clearer pathways for DTx products to improve patient outcomes, DTA will be engaging members in additional advocacy efforts. Connect with us if you would like to be involved in these efforts.
Special thanks go to DTA Resource Partner, Susan Stoner Zook, Mason Street Consulting was instrumental in planning and executing this conference.
By Andrey Ostrovsky & Everett Crosland
In August 2020, the nation’s largest health insurer, the Centers for Medicare and Medicaid Services (CMS), proposed its Medicare Coverage of Innovative Technologies (MCIT), which intended to provide national Medicare coverage on the same day as Food and Drug Administration (FDA) market authorization for breakthrough devices. The Breakthrough Devices Program at the FDA accelerates the approval or clearance process for certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Building on the Breakthrough Devices Program, MCIT could have shortened the time to market for breakthrough-designated devices (BDD) that could meaningfully improve patients’ lives.
After the transition to the Biden administration in November 2021, CMS repealed MCIT, one of the few helpful Trump-era rules. But after sharp industry and provider criticism, CMS announced listening sessions to get feedback on a replacement for MCIT called Transitional Coverage for Emerging Technologies (TCET).
In a STAT article I (A.O.) wrote last year, I argued that MCIT could meaningfully help eliminate disparities in treatment for Medicare beneficiaries and balance accountable chronic disease management with the need for social distancing in the Covid-19 era and beyond. Whether it’s called MCIT or TCET, it is imperative that CMS create an expeditious process to cover innovative devices that benefit Medicare patients and close health equity gaps, one of the main priorities of the CMS administrator, Chiquita Brooks-LaSure.
We have conducted an analysis to help CMS design TCET so that it can reduce inequities in reimbursement for BDDs, especially those focused on brain health, one of the main priorities of President Biden according to his State of the Union (SOTU) address.
To characterize and quantify the disparities against brain health in the approval and coverage of BDDs, we performed a Google search for Expedited Access Pathway (EAP) and BDDs beginning in 2015 through March 2022. We aggregated the findings into a spreadsheet and categorized the BDDs that were FDA approved or cleared, were reimbursed by CMS, whether they were digital therapeutics or diagnostics, whether they focused on behavioral health, and, as a comparator, whether they focused on cardiac health. As an external source of validation, we referenced this list against that of the Medical Device Manufacturers Association, with comparable results.
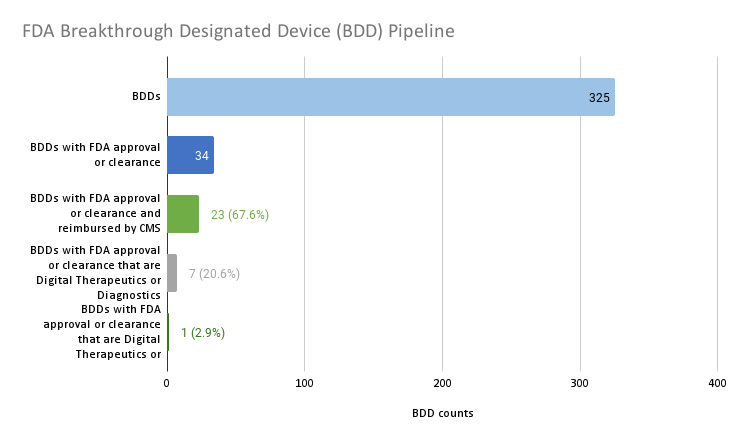
Source: Author analysis.
To date, 325 devices have been granted breakthrough designation. This analysis highlights that one of the challenges that MCIT was intended to overcome — the bottleneck of determining whether or not Medicare should cover FDA cleared or approved BDDs — is not much of an impediment to innovations reaching the market. Only 34 of the 325 BDDs, or about 1 in 10, gained FDA market authorization. And the majority, or 23 (67.6%) of the 34 BDDs with FDA market authorization, have Medicare reimbursement. There does not appear to be much of a backlog of BDDs that have achieved FDA market authorization but failed to achieve coverage.
Yet a critical issue remains that TCET could solve: There is a growing disparity in reimbursement of one class of product versus another and in reimbursing treatment and diagnosis of one disease state versus another. Only seven (20.6%) of the 34 BDDs with FDA market authorization are digital therapeutics or diagnostics. The single (2.9%) digital diagnostic that is reimbursed is for a somatic or physical condition, while the remaining digital therapeutic and diagnostics are not reimbursed and they focus on behavioral health or neurological specialties.
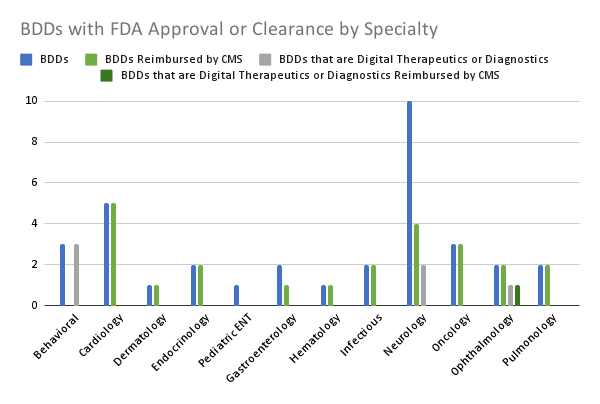
Source: Author analysis.
Digging deeper into the data, we found even more profound product class and disease state disparities. The vast majority (19 of 20, 95%) of the FDA approved or cleared BDDs that are in somatic or physical health specialties for adults are reimbursed by Medicare. Only one of the 20 (5%) of these devices was a digital therapeutic or diagnostic and it was reimbursed.
In contrast, only a fraction (4 of 13, 30.7%) of the FDA approved or cleared BDDs that are in behavioral health or neurology specialties are reimbursed by Medicare. Five of the 13 (38.5%) of these devices are digital therapeutics or diagnostics and none of them are reimbursed.
Five out of five (100%) FDA approved or cleared BDDs focused on cardiology are reimbursed by Medicare. Zero out of five (0%) FDA approved or cleared BDDs focused on behavioral health or neurology are reimbursed by Medicare. Put another way, CMS is prioritizing healing heart valves over bolstering brain health.
Digital therapeutics and diagnostics may enable patients and families to overcome barriers inherent to brick and mortar care such as transportation issues, long wait lists, and systemic racism among providers. CMS should ensure that breakthrough-designated digital therapeutics and diagnostics have an accelerated pathway to reimbursement through TCET so that the rule does not conflict with the intent of the Mental Health Parity and Addiction Equity Act, the CMS Administrator Brooks-LaSure’s focus on closing health equity gaps, and President Biden’s SOTU charge to improve brain health.
For CMS to avoid violating mental health parity laws and going against its own priorities, it will need to reconcile its historical critiques of MCIT. Most notably CMS leaders are concerned that under MCIT, CMS could have covered devices without adequate evidence to treat the Medicare population.
One way to address Medicare-specific evidence needs is to grant provisional coverage and reimbursement for BDDs with FDA market authorization during the transitional coverage period while the manufacturer develops additional evidence specific to Medicare populations. This approach can help balance equitable access to care with the need for evidentiary support.
CMS leaders’ concerns about MCIT’s evidence limitations in Medicare populations bring up another impediment to this administration reaching its equity goals. Commercial, Medicaid, and exchange insurance carriers rely on Medicare coverage determinations for many of their own medical policy decisions. Devices that fail to get Medicare coverage will lack the HCPCS codes needed for coverage with other payers. This means that BDDs with FDA market authorization serving children and adults under 65, especially those in poverty, are systematically not getting covered.
To address the Medicare coverage roadblock for commercial, Medicaid, and exchange insurance carriers, CMS could create modifiers for HCPCS codes that designate which populations certain codes could be applied to, such as pediatric populations and adults under 65.
Including HCPCS code modifiers may not be enough for TCET to address CMS’s current coverage disparities. TCET should call for the Center on Medicare to improve internal processes so that there is more expertise and prioritization on brain health and non-Medicare populations. With subject matter experts on these topics in the Center for Medicaid and CHIP Services (CMCS) and the Center for Medicare and Medicaid Innovation (CMMI), TCET should require that leaders in the Center on Medicare more routinely collaborate with CMCS and CMMI leaders on coverage determinations.
Despite all of these critiques, we have to give the Center for Medicare credit because they did finally open the door for digital therapeutics coding by approving the HCPCS Level II code A9291 for “Prescription digital behavioral therapy, fda cleared, per course of treatment.” However, Medicare still doesn’t reimburse this code and this code lacks the specificity needed for most commercial insurers to systematically reimburse all brain health focused digital therapeutics.
Medicare also has made productive strides in providing reimbursement for the new CPT code 989X5 for “Remote therapeutic monitoring,” which could be used by some digital therapeutics for reimbursement. However the reimbursement amount is anemic and is mostly limited to musculoskeletal and respiratory indications.
This analysis demonstrates that CMS’s coverage processes demonstrate product class and disease state disparities. These disparities contradict mental health parity statute, CMS Administrator Brooks-LaSure’s focus on equity, and President Biden’s charge to improve brain health. TCET can help close these gaps by granting provisional coverage for BDDs with FDA market authorization with evidence development, creating modifiers for HCPCS codes for non-Medicare populations, and requiring the Center on Medicare to incorporate experts from CMCS and CMMI into coverage determinations.
Andrey Ostrovsky is a pediatrician, managing partner at Social Innovation Ventures, former chief medical officer for the Center for Medicaid and CHIP Services, and a strategic advisor to the Digital Therapeutics Alliance.
Everett Crosland is the Chief Commercial Officer at Cognito Therapeutics and board member of the Digital Therapeutic Alliance.
March 24, 2022 – Arlington, VA – The Digital Therapeutics Alliance (DTA) is leading policy and advocacy efforts to expand adoption, coverage, and access to clinically validated digital therapeutics (DTx). During a critical time, this innovative category of medicine is addressing unmet needs and major gaps in healthcare. The pandemic and subsequent challenges have resulted in many patients unable to access much needed care – either due to geographic limitations, cultural and language barriers, well-documented disparities, health condition severity, or demand far exceeding the supply of qualified clinicians.
DTA is working with policymakers to support the wider integration of DTx therapies across the healthcare ecosystem to ensure these evidence-based treatments get into the hands of those who need them most.
DTA’s current advocacy efforts in the United States are focused on:
By engaging directly with federal agency officials and submitting comments on proposed rules and regulations, DTA is working to ensure members and the patients they serve are considered in policy decisions.
As a part of these efforts, this week DTA submitted comments related to two Food and Drug Administration draft guidances:
Additional recent responses from DTA include:
February 28, 2022 – DTA responds to the White House Office of Science and Technology Policy (OSTP) Request for Information (RFI) on Strengthening Community Health Through Technology (87 FR 492), published January 5, 2022.
February 28, 2022 – DTA responds to the Food and Drug Administration’s ‘Considerations for the Use of Real-World Data and Real-World Evidence To Support Regulatory Decision-Making for Drug and Biological Products,’ (FDA-2021-D-1214) published December 9, 2021.
February 28, 2022 – DTA responds to the Food and Drug Administration’s ‘Real-World Data: Assessing Registries to Support Regulatory Decision-Making for Drug and Biological Products,’ (FDA-2021-D-1146) published November 30, 2021.
January 15, 2022 – DTA responds providing an overview of efforts to ensure product safety and reliability for the White House Office of Science and Technology Policy (OSTP) Request for Information on public and private uses of biometric technologies (86 FR 56300), published October 8, 2021.
November 29 , 2021- DTA responds to the Agency for Healthcare Research and Quality’s (AHRQ) draft technical brief “Evaluation of Mental Health Mobile Applications.” (86 FR 28601), published October 20, 2021.
November 26, 2021 – DTA responds to Food and Drug Administration’s ‘Notice to Public of Website Location of CDRH Fiscal Year 2022 Proposed Guidance Development,’ (FDA-2012-N-1021), published October 26, 2021.
DTA keeps members informed about all relevant federal actions in the U.S. as well as provides opportunities for members to directly engage in advocacy efforts through its US Policy Task Group, member newsletter, and Slack community. Connect with us if you would like to be involved in these efforts.
Tuesday, March 22, 2022 – Arlington, VA – This year, The Digital Therapeutics Alliance (DTA) is leading initiatives focused on three priorities — at the international, national, and regional levels — to drive the industry forward.
DTA members and Resource Partners are spearheading our efforts to transform global healthcare through seven newly launched Task Groups.
Asia Pacific Policy Task Group
Focus: This group focuses on key aspects of the Asia-Pacific policy landscape, including regulatory frameworks and national funding pathways.
DTA Member Co-Chairs:
Focus: This group is developing DTx-specific guidelines and core criteria for healthcare decision maker clinical evidence evaluation processes.
DTA Member Co-Chairs:
Resource Partners:
DTx Value Assessment & Integration Guide Task Group
Focus: This group is guiding the launch and update phases of DTA’s ‘DTx Value Assessment & Integration Guide,’ which establishes a framework to consistently evaluate and benchmark DTx products.
DTA Member Co-Chairs:
Resource Partner:
Focus: This group focuses on key aspects of the European policy landscape, including Health Technology Assessment (HTA) frameworks, national funding, and patient access pathways.
DTA Member Co-Chairs:
Public Relations & Communications Task Group
Focus: This group is developing resources to promote broader DTx awareness and understanding, in addition to addressing industry-level challenges.
DTA Member Co-Chairs:
US Commercialization & Reimbursement Task Group
Focus: This group is working to establish payment pathways in the United States by addressing coding and coverage issues, in addition to developing product commercialization resources.
DTA Member Co-Chairs:
Resource Partners:
Focus: This group is focused on DTA’s ongoing work related to U.S. legislation and regulatory efforts with federal agencies.
DTA Member Co-Chairs:
Resource Partners:
As DTA continues to lead the digital therapeutic industry, these collective efforts will be instrumental in providing patients and caregivers, clinicians, policymakers, and healthcare decision makers with reliable resources to meaningfully integrate DTx products into practice to improve clinical and health economic outcomes globally.
About DTA
The Digital Therapeutics Alliance (DTA) is a global non-profit trade association of industry leaders and stakeholders with the mission of broadening the understanding, adoption, and integration of digital therapeutics into healthcare. DTA works to enable expanded access to high quality, evidence-based digital therapeutics for patients, clinicians, and payors to improve clinical and health economic outcomes. To learn more, please visit: www.dtxalliance.org or follow us on LinkedIn and Twitter.